Publications
Google Scholar Page
Issued Patents
Pre-Prints:
Levinn, C.M.; Pluth, M.D. “Learning from Our Mistakes: We Have a Scientific and Fiscal Obligation to Publish Failed Results.” ChemRxiv 2020. [10.26434/chemrxiv.13141283.v1]
Published:
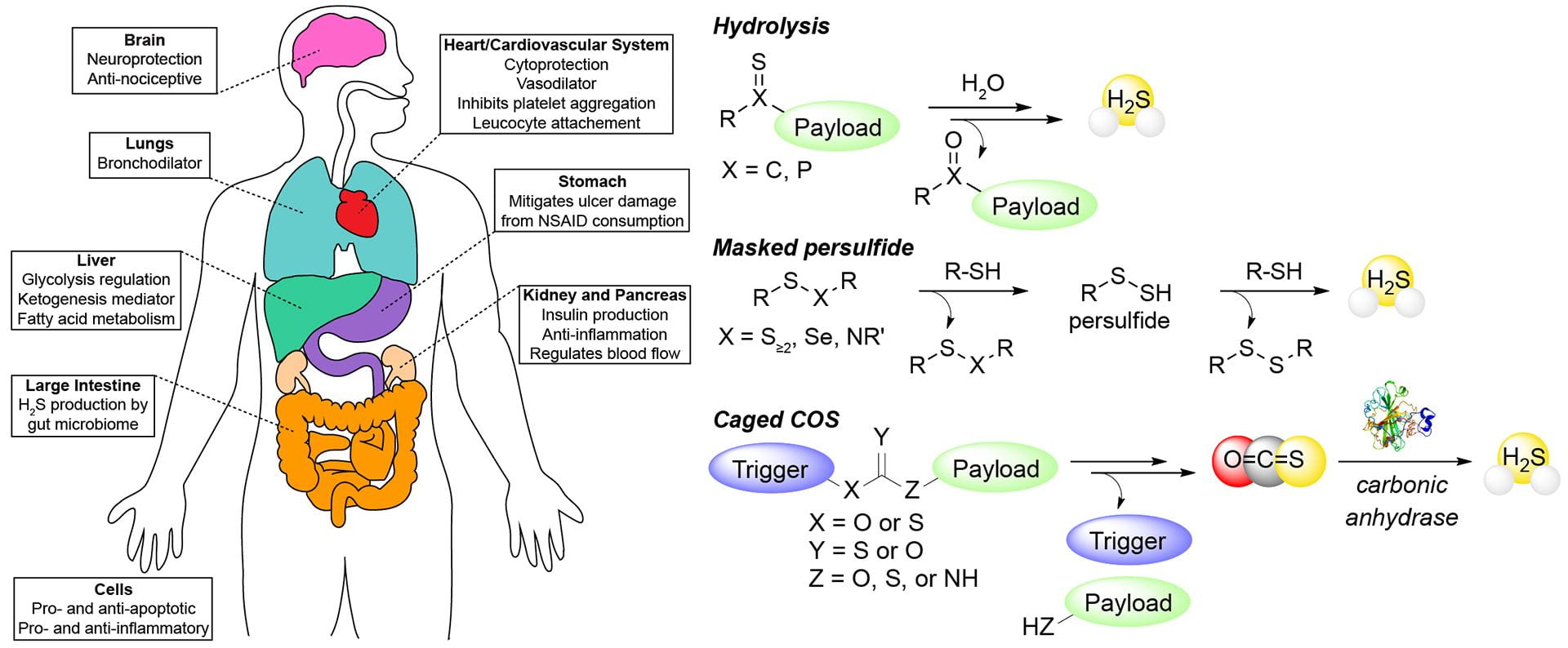
- Davis, A.G.; Pluth, M.D. “Protic Small Molecule Bioregulators.” Redox Biology 2025, 103921. [10.1016/j.redox.2025.103921]

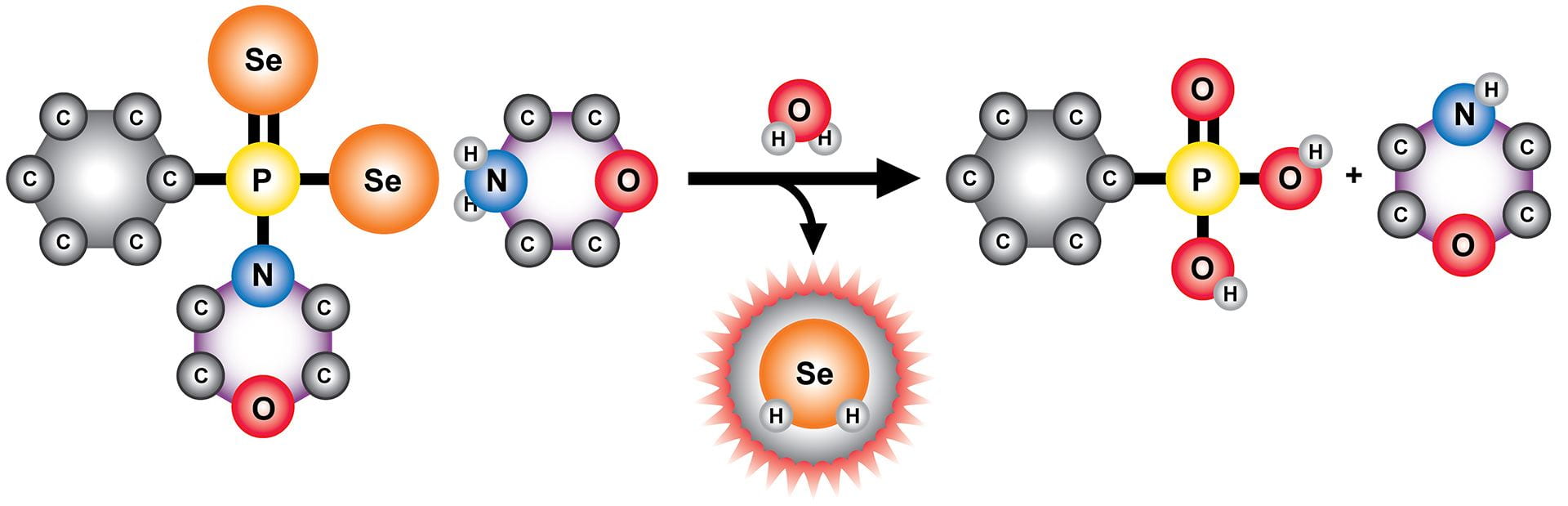
- Li, K.; Sattler, A.J.; Zakharov, L.N.; Pluth, M.D. “Synthetic Motifs for Understanding Lewis Acid Interactions with Persulfides and Thioselenides.” Angew. Chem. Int. Ed. 2025 early view. [10.1002/anie.202520417]
– Selected as VIP article

- Pluth, M.D. “Harnessing Hydrogen Cyanide (HCN): Responsive Chemical Tools for Delivering an Emerging Gasotransmitter.” Angew. Chem. Int. Ed. 2025 early view. [10.1002/anie.202521917]

- Dorit, R.D.; Li, K.; Steven, C.A.; Garcia, A.C.; Newton, T.D.; Fosnacht; K.G.; Pluth, M.D. “Cysteine-activated hydrogen selenide (H2Se) delivery from isoselenocyanates.” Chem. Commun. 2025, early view. [10.1039/D5CC05352H]

- Smith, H.M.; Lengkong, A.J.; Steven, C.A.; Stonas, A.N.; Pluth, M.D. “Thiophene Esters Are Not Selective for H2S and Undergo Thiol and Esterase Cleavage.” ACS Chem. Biol. 2025, ASAP article. [10.1021/acschembio.5c00527]

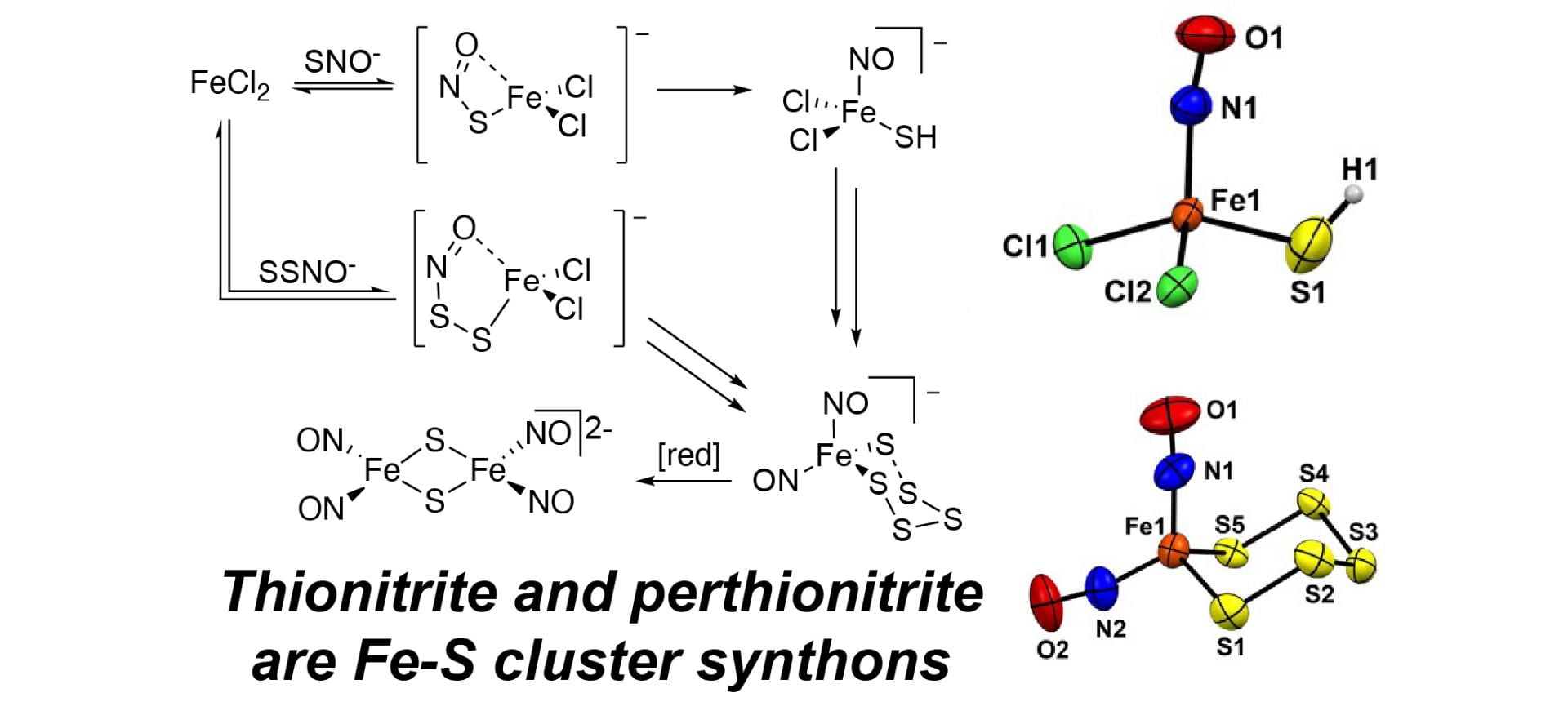
- Li, K.; Hernandez, J.F.; Piepgras, G.W.; Zakharov, L.N.; Pluth, M.D. “Understanding Reactive Sulfur Species Storage from Hybrid S/N Crosstalk Species: Perthionitrite (SSNO−) and Thionitrite (SNO−) Activation by a Mononuclear Nonheme Fe(II) Complex.” Inorg. Chem. 2025, 64(26), 13061-13068. [10.1021/acs.inorgchem.5c01177]

- Sherbow, T.J.; Smith, H.M.; Li, K.; Zakharov, L.N.; Pluth, M.D.; Pluth, M.D. “Investigating Molybdenum and Tungsten Carbonyls as a Bridge Between Elemental Chalcogens and CO: Formation of Carbonyl Sulfide and Selenium Complexes.” Inorg. Chem. 2025, 64(25), 12651-12657. [10.1021/acs.inorgchem.5c01261]

- Moaven, S.; Vander Griend, D.A.; Johnson, D.W.; Pluth, M.D. “Expanding the Toolbox for Supramolecular Chemistry: Probing Host-Guest Interactions with in situ FTIR Spectroscopy for Structural and Dynamic Insights.” Chem. Sci. 2025, 16, 10282-10288. [10.1039/D5SC01329A]
– Chem. Sci. Hot Article, 2025

- Davis, A.G.; Zakharov, L.N.; Pluth, M.D. “Probing the Reversible Binding of Anionic Reactive Sulfur and Nitrogen Species in Imidazolium Receptors with Directional C–H Hydrogen Bonds.” Inorg. Chem., 2025, 64(15), 7774-7783. [10.1021/acs.inorgchem.5c00821]

- Davis, A.G.; Pluth, M.D. “Experimental Insights into the Formation, Reactivity, and Crosstalk of Thionitrite (SNO–) and Perthionitrite (SSNO–).” Angew. Chem. Int. Ed., 2025, 64(1), e202413092. [10.1002/anie.202413092]

- Wade Wolfe, M.M.; Pines, J.E.; Sosa, A.R.; Pluth, M.D. “Exploring the Reactivity of Electrophilic Organic Carbonates and Thiocarbonates as Vehicles to Convert Hydrosulfide into COS and CS2.” J. Org. Chem., 2024, 89(21), 16004–16009. [10.1021/acs.joc.4c01690]

- Garcia, A.C.; Shavlik, M.; Harms, M.J.; Pluth, M.D. “Structural Deformations in Cucurbit[n]urils: Analysis, Host-Guest Dependence, and Automated Ellipticity Measurements using ElliptiCB[n].” Chem. Eur. J., 2024, 30(57), e202401981. [10.1002/chem.202401981] [GitHub]

- Li, K.; Zakharov, L.N.; Pluth, M.D. “Synthesis, Characterization, and Reactivity of a Synthetic End-on Cobalt (II) Alkyl Persulfide Complex as a Model Platform for Thiolate Persulfidation.” J. Am. Chem. Soc., 2024, 146(31), 21999-22007. [10.1021/jacs.4c07276]

- Fosnacht, K.G.; Sharma, J.; Champagne, P.A.; Pluth, M.D. “Transpersulfidation or H2S Release? Understanding the Landscape of Persulfide Chemical Biology.” J. Am. Chem. Soc., 2024, 146(27), 18689-18698. [10.1021/jacs.4c05874]

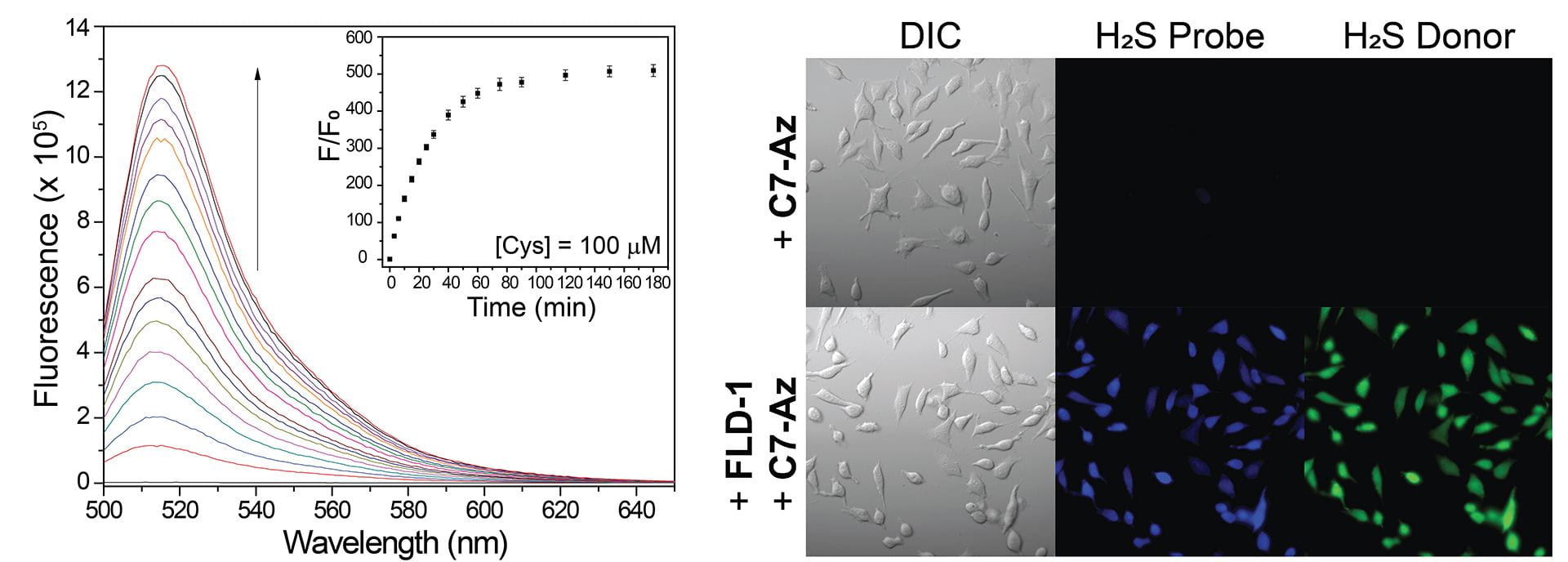
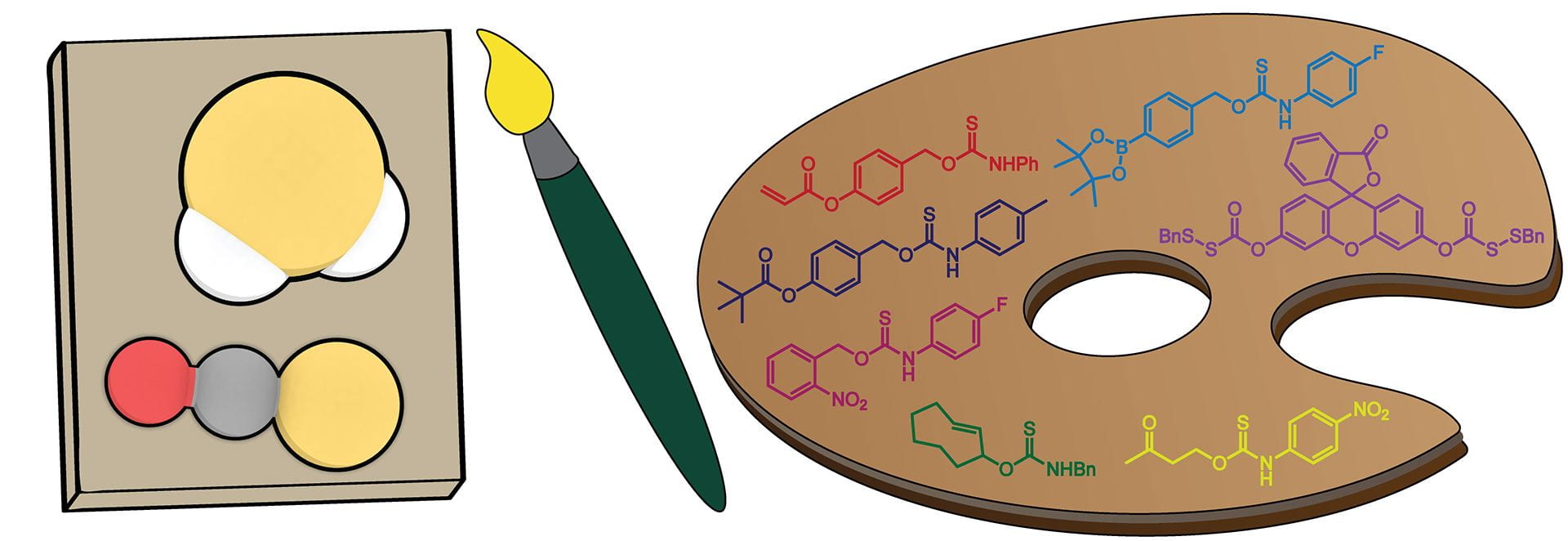
- Fosnacht, K.G.; Dorogin, J.; Jefferis, P.M.; Hettiaratchi, M.H.; Pluth, M.D. “An Expanded Palette of Fluorescent COS/H2S-Releasing Donors for H2S Delivery, Detection, and In Vivo Application.” Angew. Chem. Int. Ed., 2024, 63(24), e202402353. [10.1002/anie.202402353]
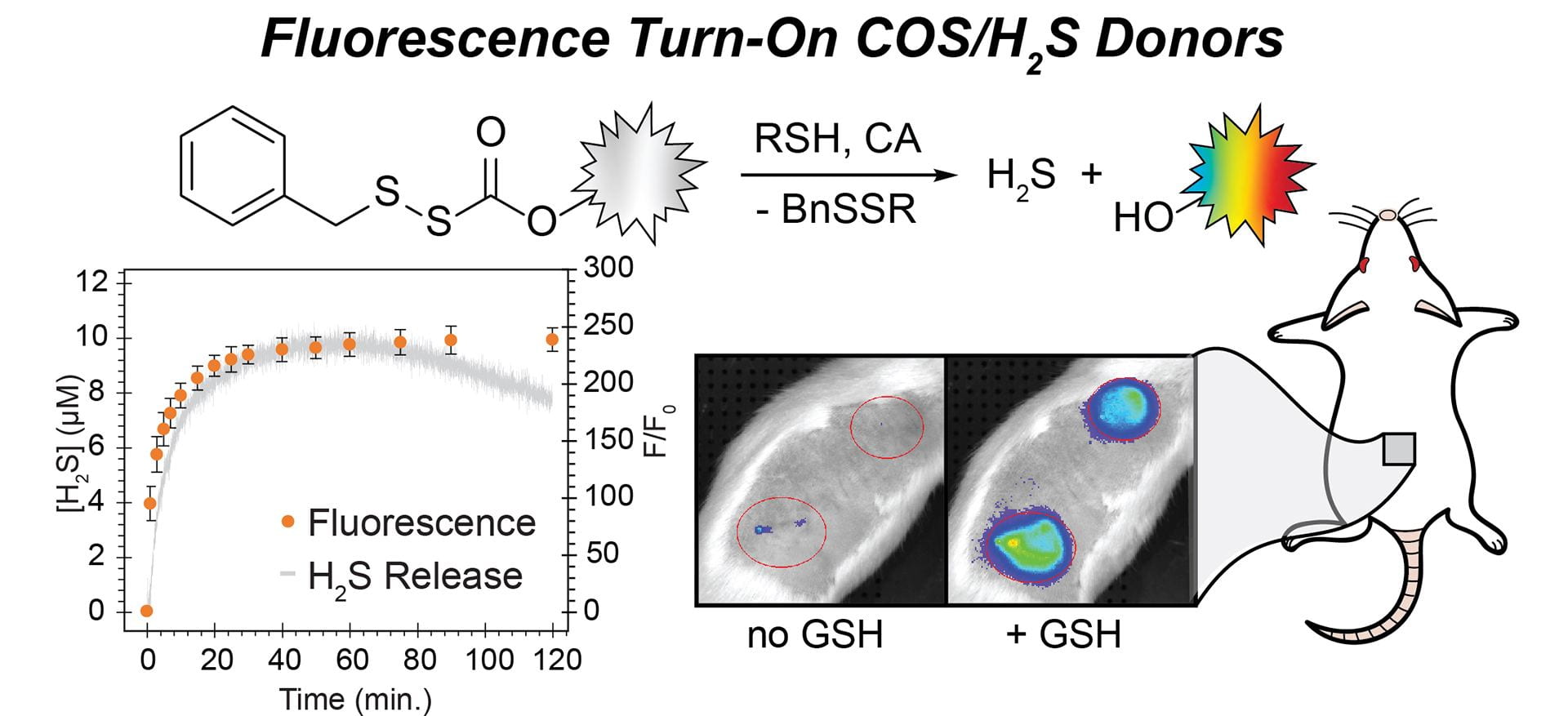
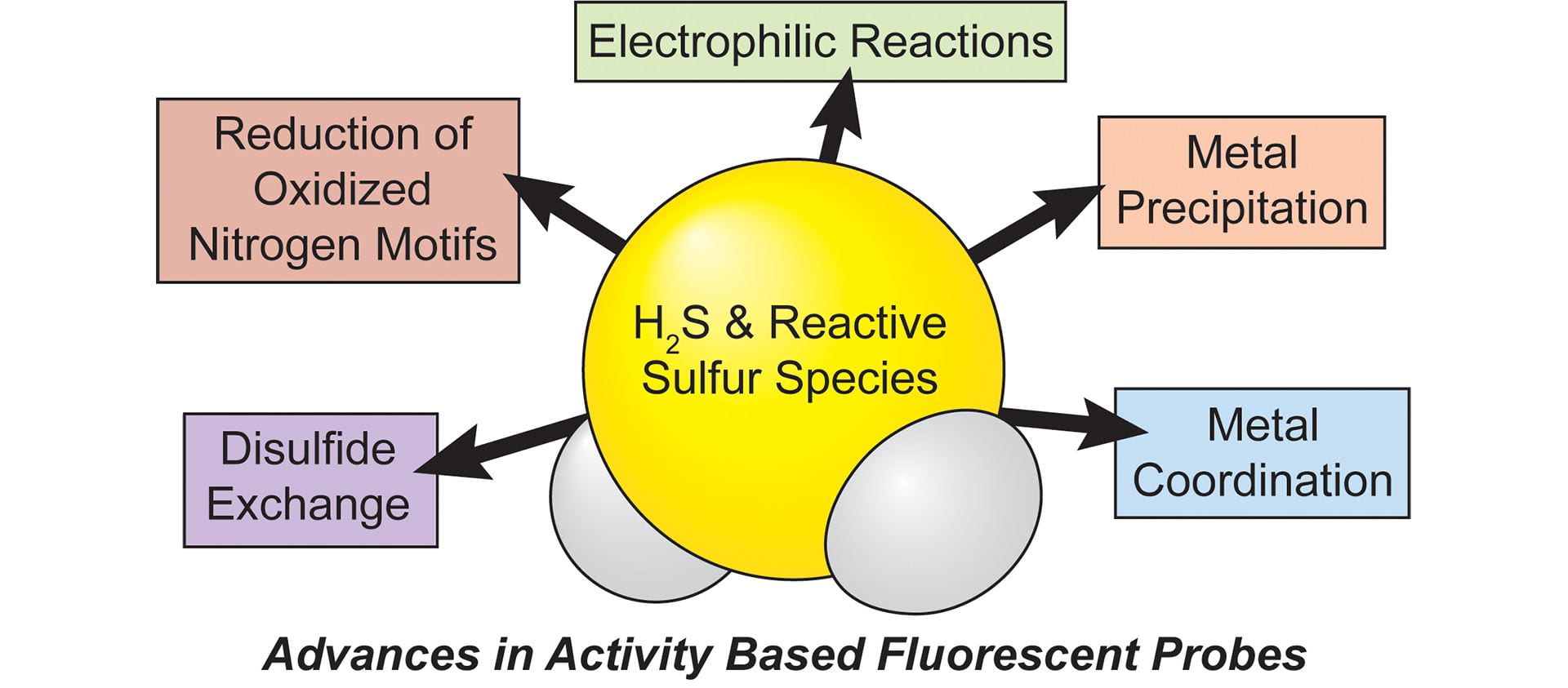
- Fosnacht, K.G.; Pluth, M.D. “Activity-Based Fluorescent Probes for Hydrogen Sulfide and Related Reactive Sulfur Species.” Chem. Rev., 2024, 124(7), 4124–4257. [10.1021/acs.chemrev.3c00683]
- Davis, A.G.; Zakharov, L.N.; Pluth, M.D. “Reversible Hydrosulfide (HS–) Binding using Exclusively C–H Hydrogen Bonding Interactions in Imidazolium Hosts.” Inorg. Chem., 2024, 63(6), 3057–3062. [10.1021/acs.inorgchem.3c03922]

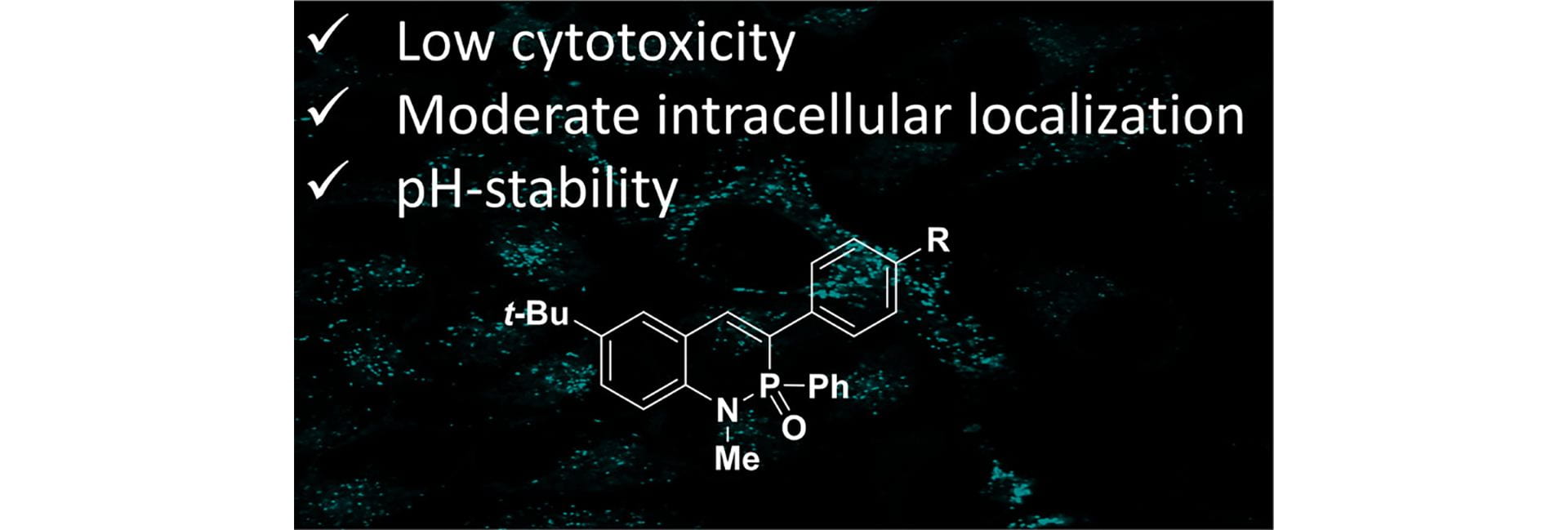
- Bard, J.P.; Bolton, S.G.; Howard, H.J.; McNeill, J.N.; de Faria, T.P.; Zakharov, L.N.; Johnson, D.W.; Pluth, M.D.; Haley, M.M. “2-λ5-Phosphaquinolin-2-ones as Non-cytotoxic, Targetable, and pH-Stable Fluorophores.” J. Org. Chem., 2023, 81(21), 15516-15522. [10.1021/acs.joc.3c01927]
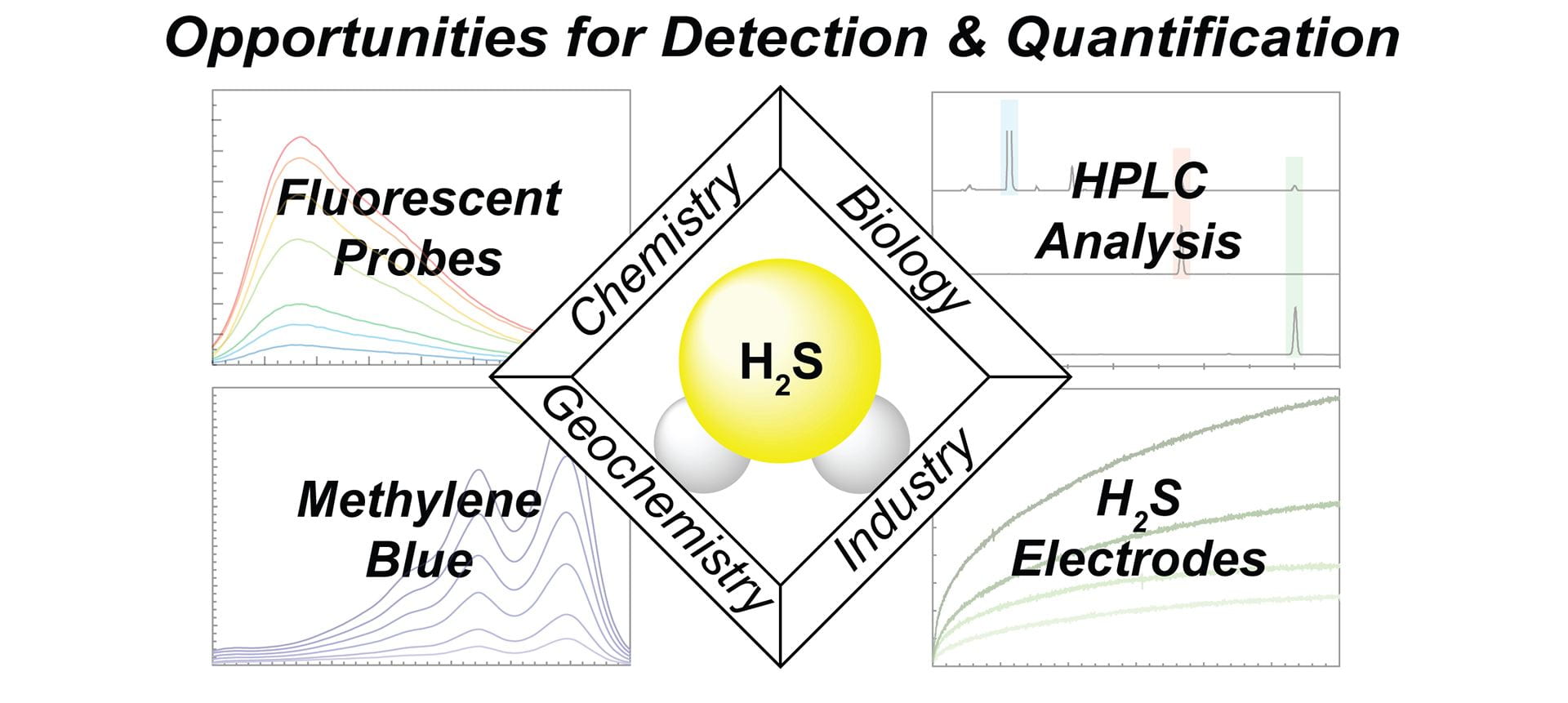
- Smith, H.M.; Pluth, M.D. “Advances and Opportunities in H2S Measurement in Chemical Biology.” JACS Au, 2023, 3(10), 2677–2691. [10.1021/jacsau.3c00427]
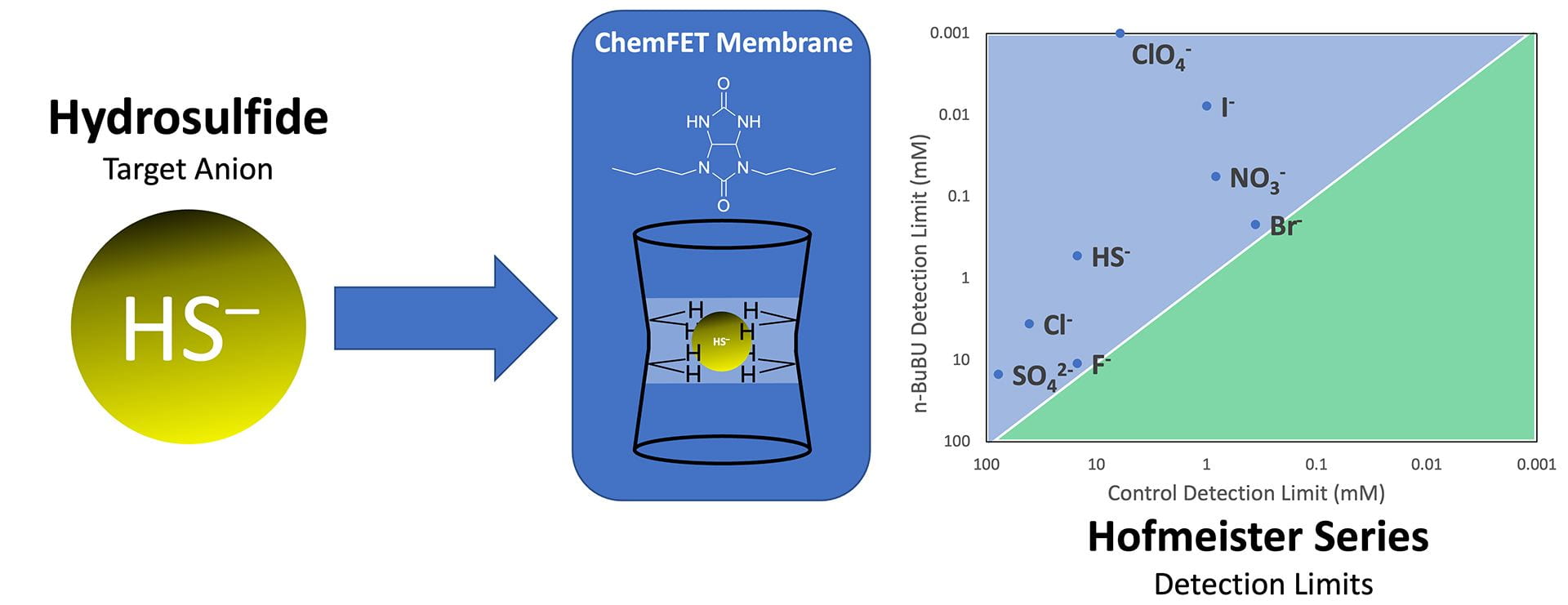
- Kuhl, G.M.; Banning, D.H.; Fargher, H.A.; Davis, W. A.; Howell, M.M.; Zakharov, L.N.; Pluth, M.D.; Johnson, D.W. “Benchmarking the Placement of Hydrosulfide in the Hofmeister Series Using a Bambus[6]uril-based ChemFET Sensor.” Chem. Sci., 2023, 14, 10273-10279. [10.1039/D3SC03616B]
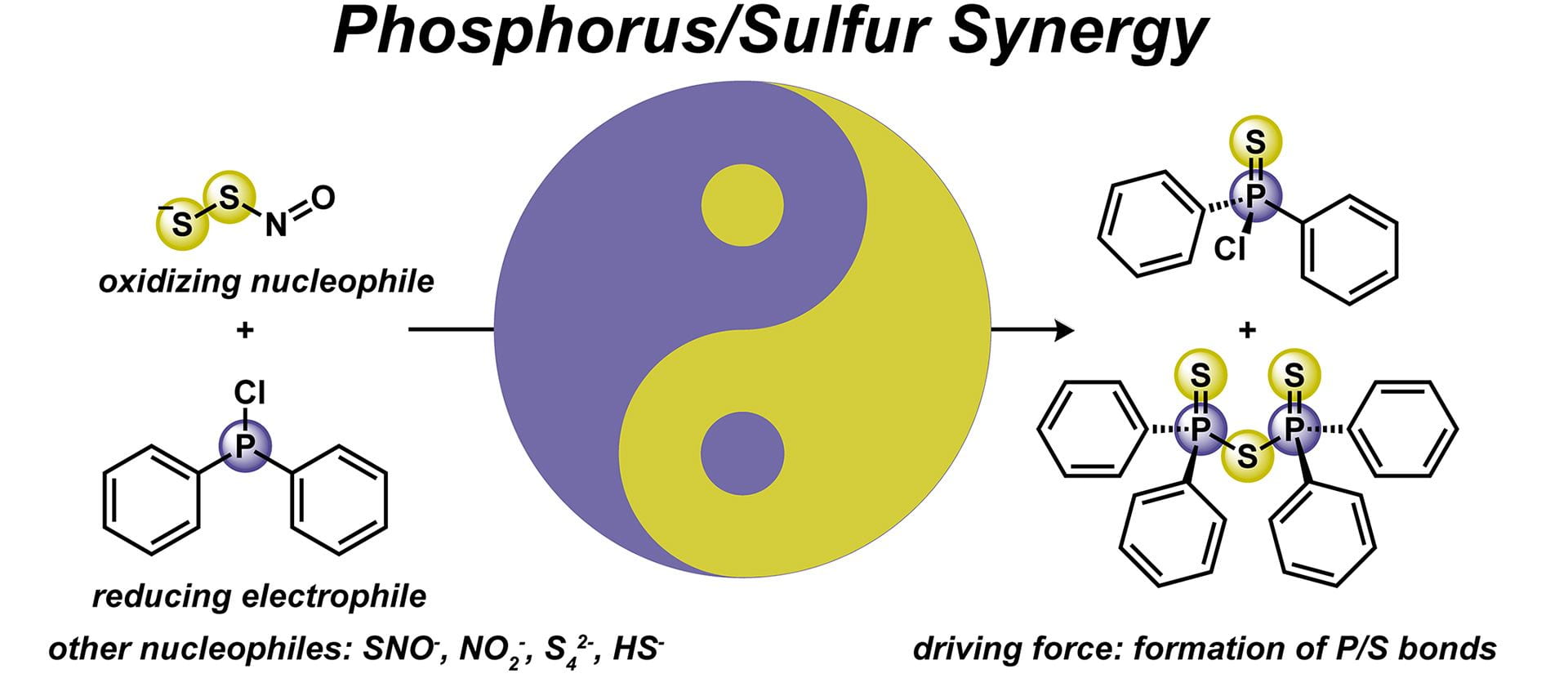
- Wade Wolfe, M.M.; Pluth, M.D. “Understanding Reactive Sulfur Species through P/S Synergy.” Inorg. Chem., 2023, 62(35), 14339-14343. [10.1021/acs.inorgchem.3c01976]
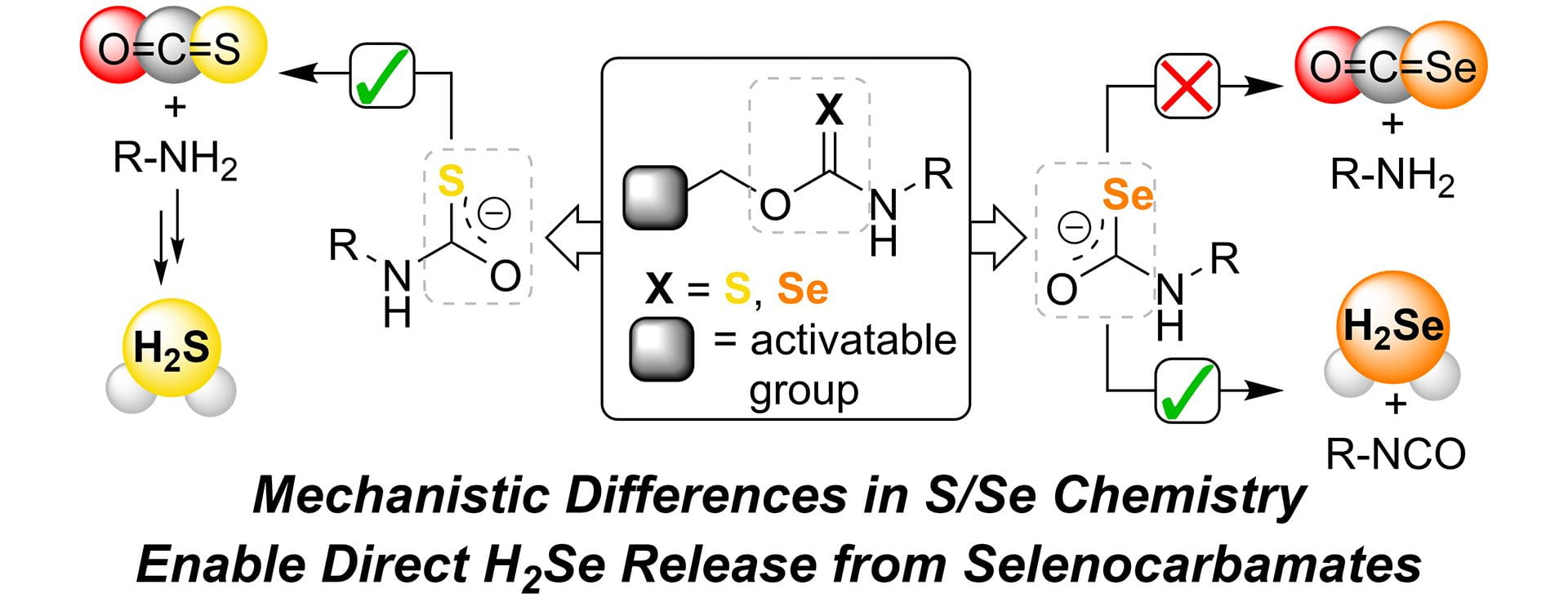
- Newton, T.D.; Li, K.; Sharma, J.; Champagne, P.A.; Pluth, M.D. “Direct Hydrogen Selenide (H2Se) Release from Activatable Selenocarbamates.” Chem. Sci., 2023, 14, 7581-7588. [10.1039/D3SC01936E]
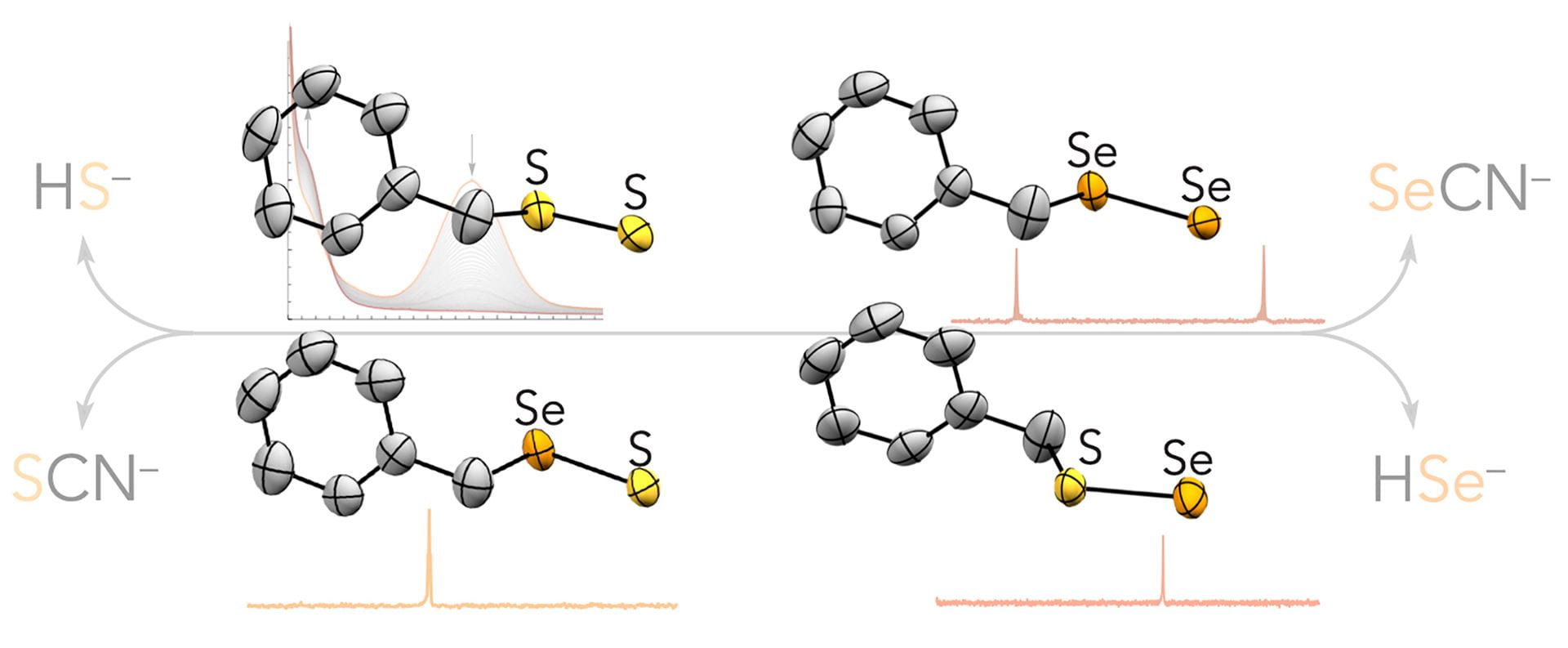
- Li, K.; Zakharov, L.N.; Pluth, M.D. “Taming the Dichalcogenides: Isolation, Characterization, and Reactivity of Elusive Perselenide, Persulfide, Thioselenide, and Selenosulfide Anions.” J. Am. Chem. Soc., 2023, 145(24), 13435-13443. [10.1021/jacs.3c03766]
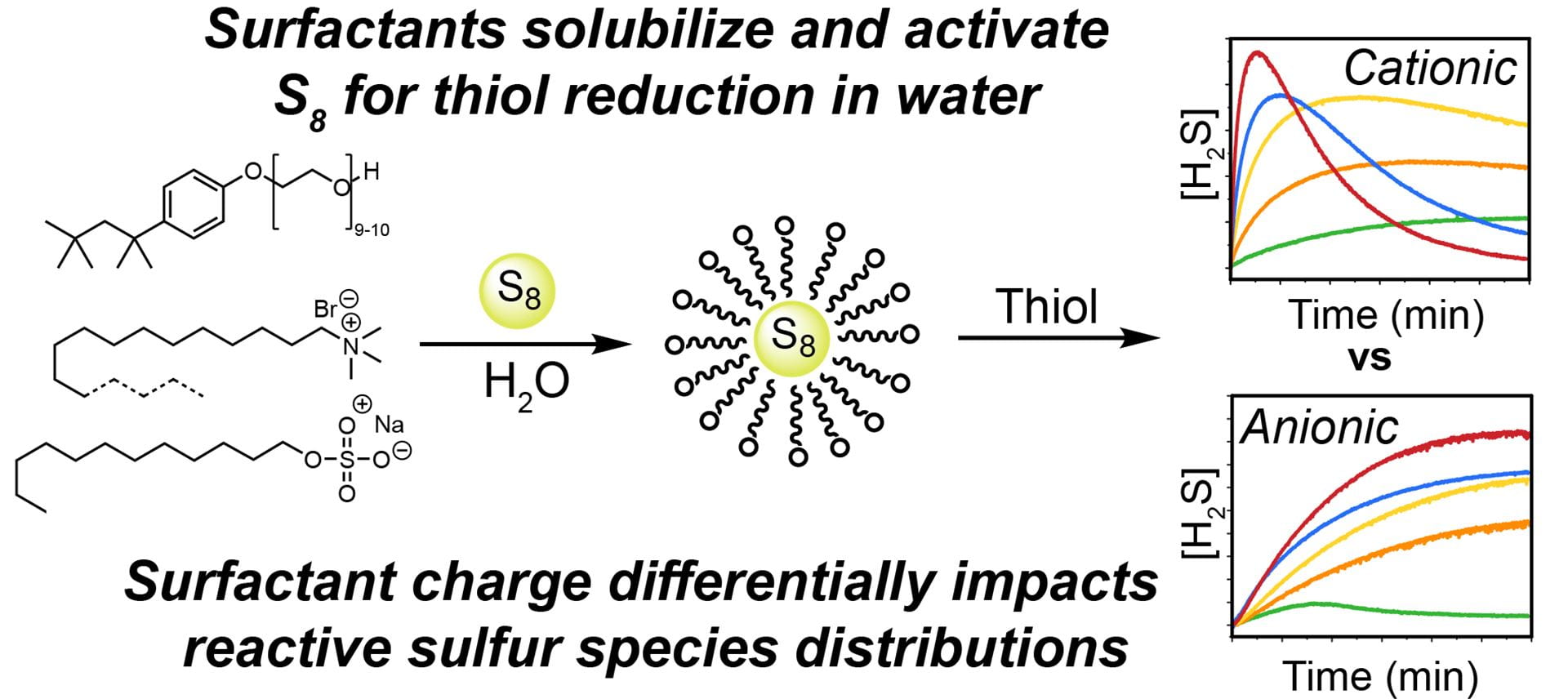
- Garcia, A.C.; Pluth, M.D. “Solubilization of Elemental Sulfur by Surfactants Promotes Reduction to H2S by Thiols.” Chem. Commun., 2023, 59, 6702-6705. [10.1039/D3CC01914D]
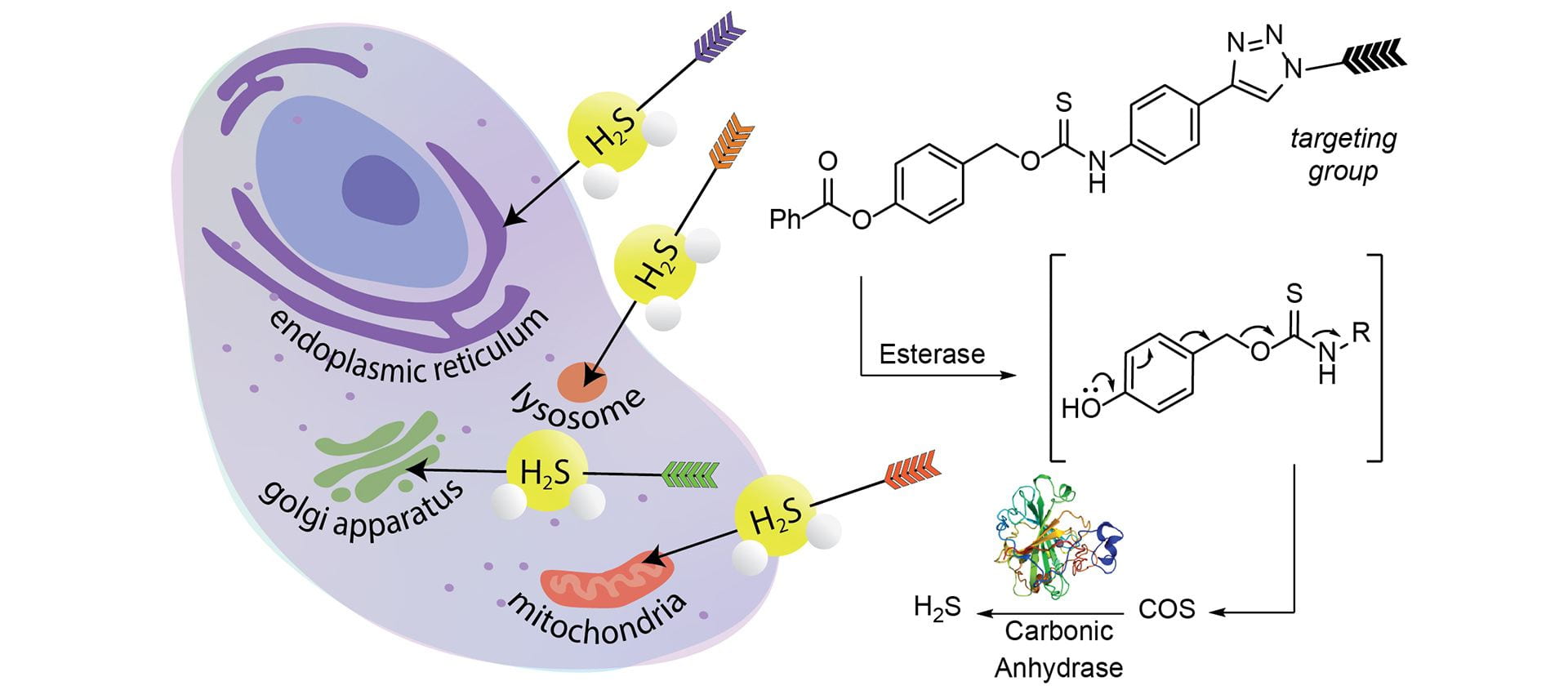
- Gilbert, A.K.; Pluth, M.D. “Subcellular Delivery of Hydrogen Sulfide Using Small Molecule Donors Impacts Organelle Stress.” J. Am. Chem. Soc., 2022, 144(38), 17651–17660. [10.1021/jacs.2c07225]
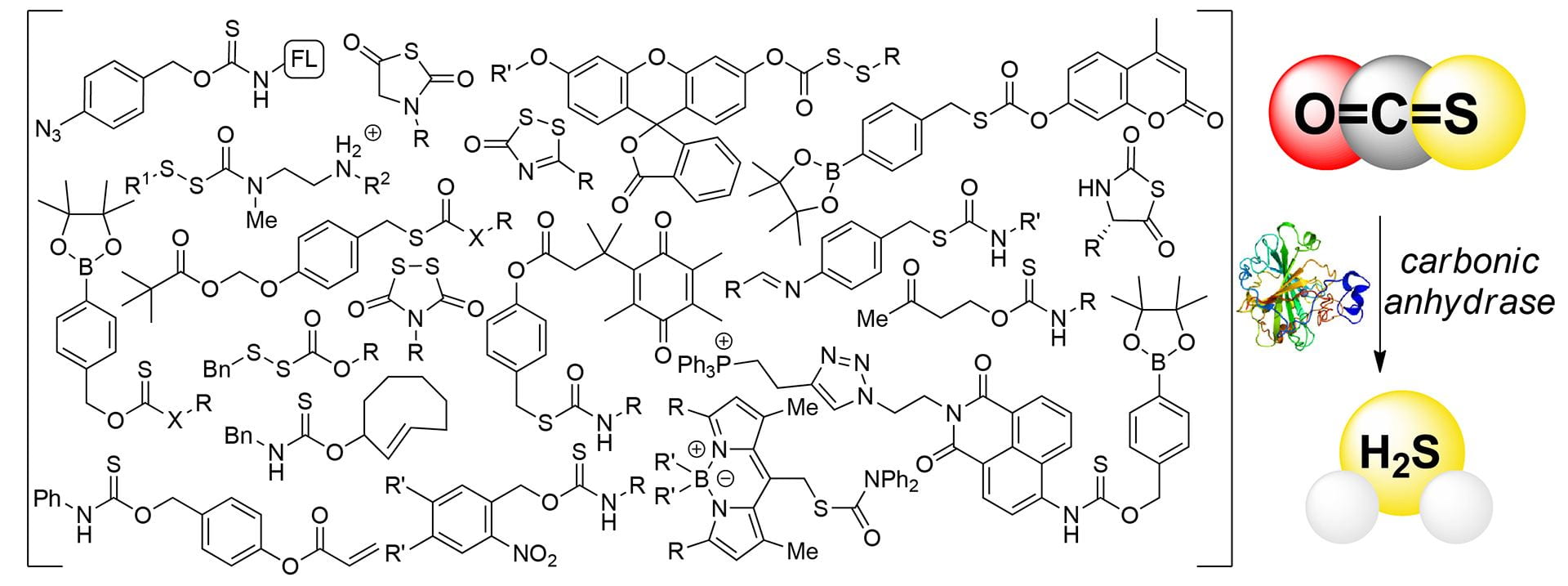
- Gilbert, A.K.; Pluth, M.D. “COS-Based H2S Donors.” in Hydrogen Sulfide: Chemical Biology Basics, Detection Methods, Therapeutic Applications, and Case Studies. (Wiley-VCH), 2022, 321-346 [10.1002/9781119799900.ch13]

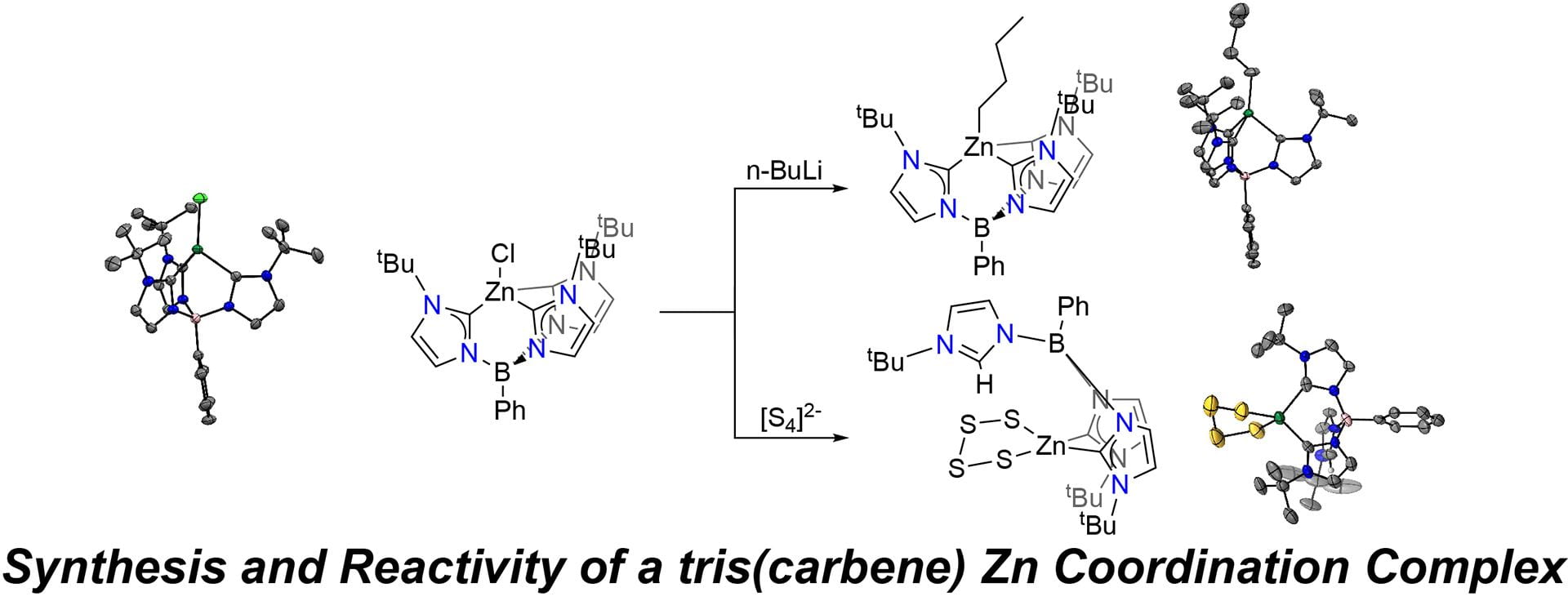
- Sherbow, T.J.; Li, K.; Zakharov, L.N.; Pluth, M.D. “Synthesis and Reactivity of a tris(carbene) Zinc Chloride Complex.” Dalton Trans. 2022, 51, 14563-14567. [10.1039/D2DT02809C]
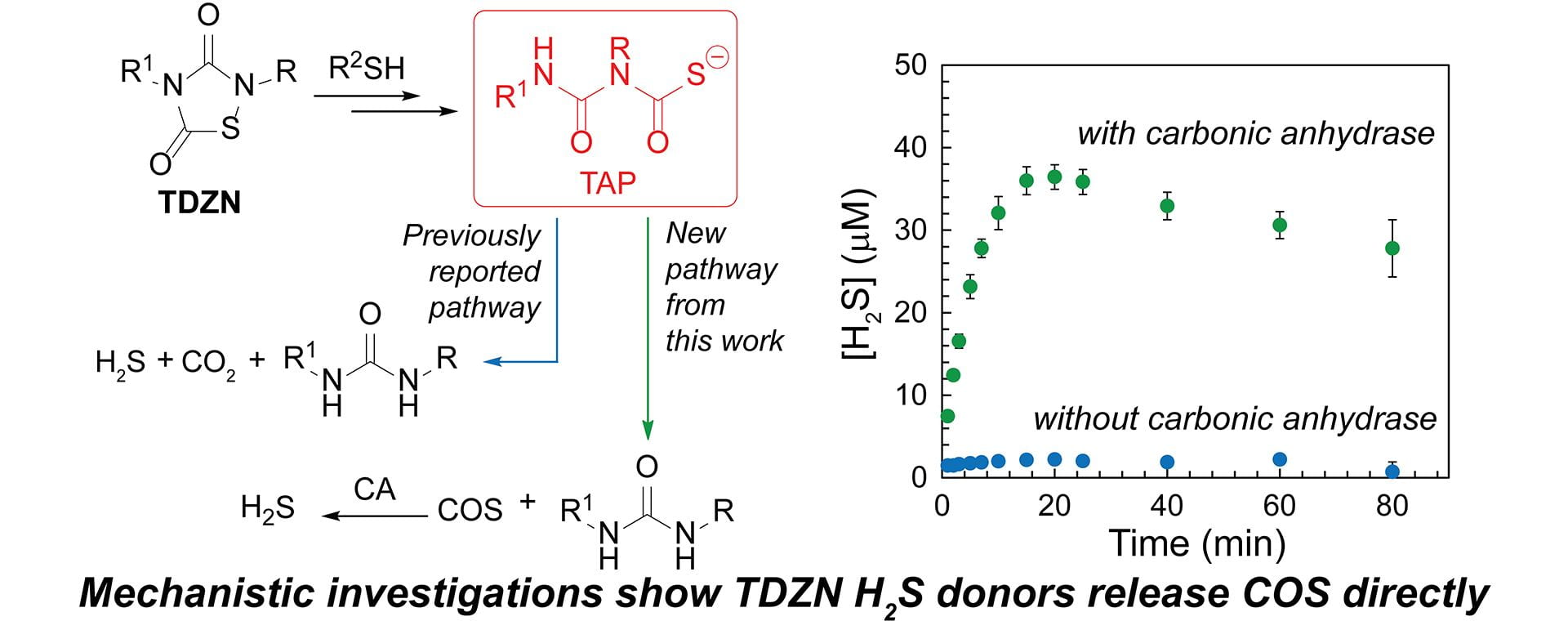
- Smith, H.M.; Pluth, M.D. “Thiol-Activated 1,2,4-Thiadiazolidin-3,5-diones Release Hydrogen Sulfide through a Carbonyl-Sulfide-Dependent Pathway.” J. Org. Chem. 2022, 87(18), 12441–12446. [10.1021/acs.joc.2c01220]
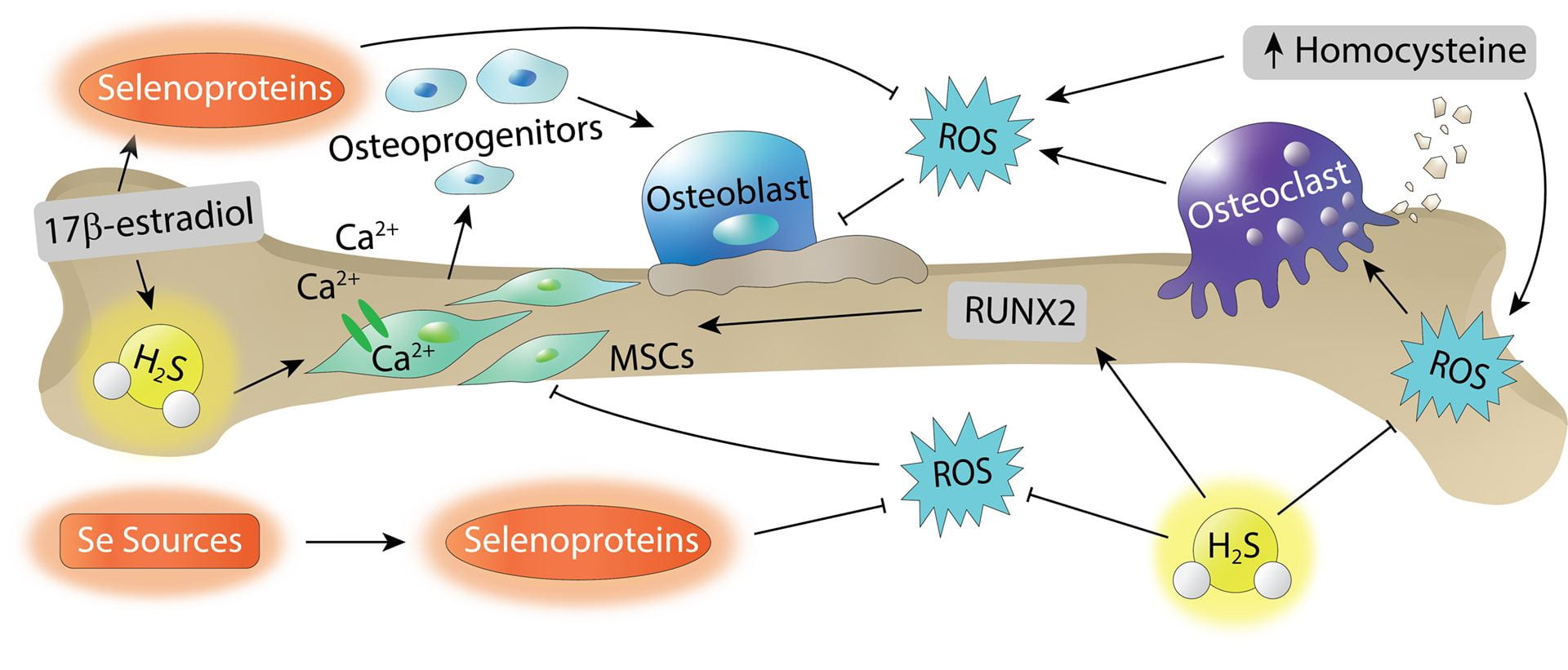
- Gilbert, A.K.; Newton, T.D.; Hettiaratchi, M.H.; Pluth, M.D. “Reactive Sulfur and Selenium Species in the Regulation of Bone Homeostasis.” Free Radic. Biol. Med. 2022, 190, 148-157. [10.1016/j.freeradbiomed.2022.08.002]
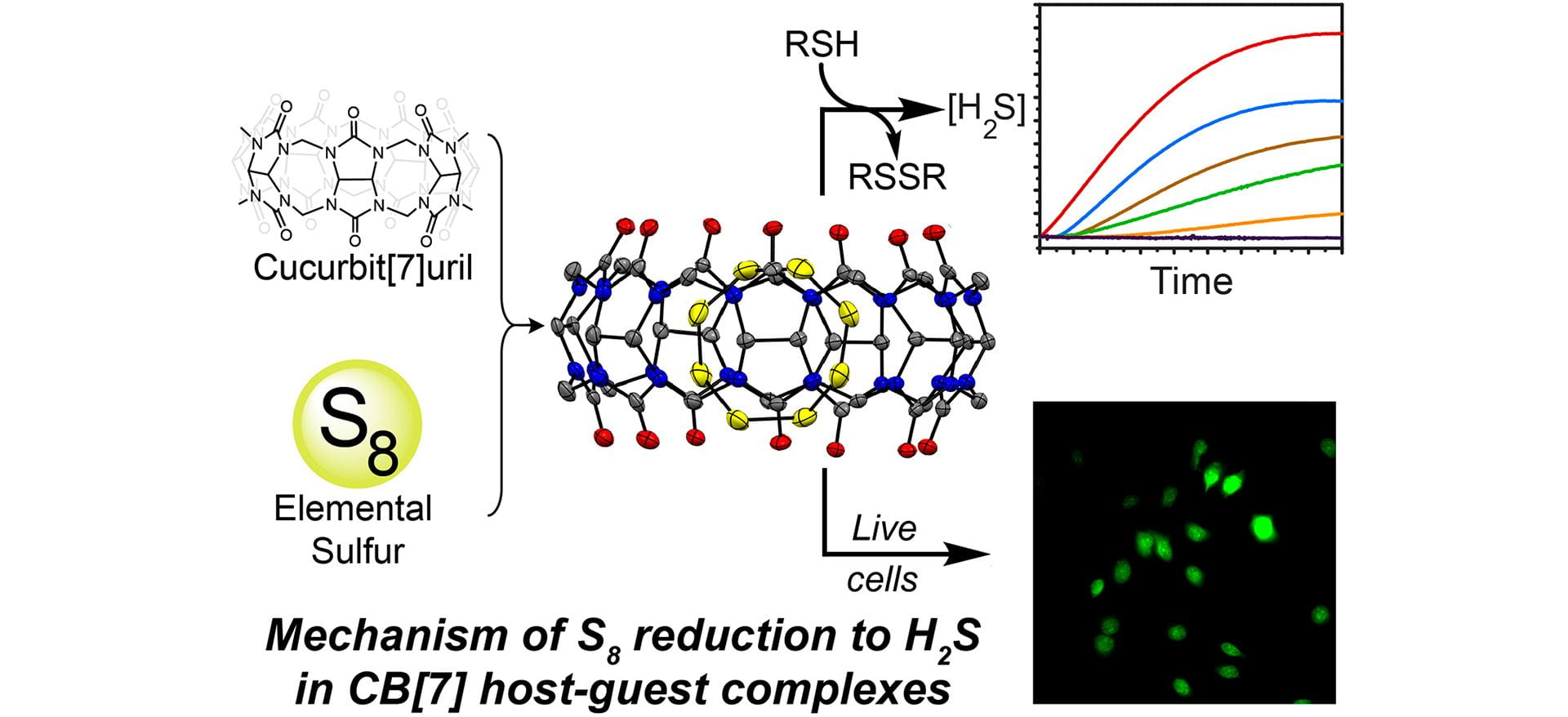
- Garcia, A.C.; Zakharov, L.N.; Pluth, M.D. “Supramolecular Activation of S8 by Cucurbiturils in Water and Mechanism of Reduction to H2S by Thiols: Insights into Biological Sulfane Sulfur Trafficking.” J. Am. Chem. Soc. 2022, 144(33), 15324-15332. [10.1021/jacs.2c06332]
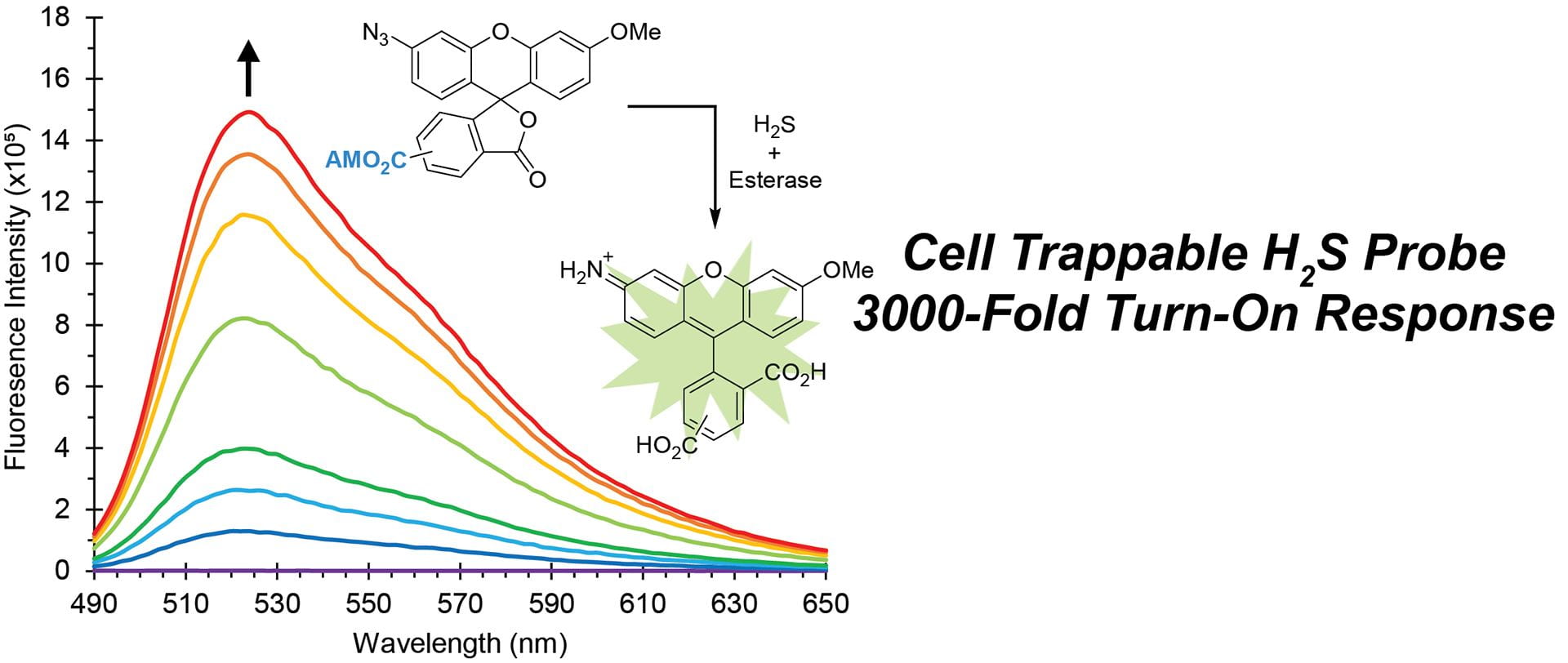
- Fosnacht, K.G.; Hammers, M.D.; Earp, M.S.; Gilbert, A.K.; Pluth, M.D. “A Cell Trappable Methyl Rhodol-Based Fluorescent Probe for Hydrogen Sulfide Detection.” Chem. Asian J. 2022, 17(16), e202200426. [10.1002/asia.202200426]
- Sherbow, T.J.; Fu, W.; Tao, L.; Britt, D.R.; Pluth, M.D. “Thionitrite (SNO–) and Perthionitrite (SSNO–) are Simple Synthons for Nitrosylated Iron Sulfur Clusters.” Angew. Chem. Int. Ed. 2022, 61(30), e202204570. [10.1002/anie.2022045702]
– Selected as VIP article
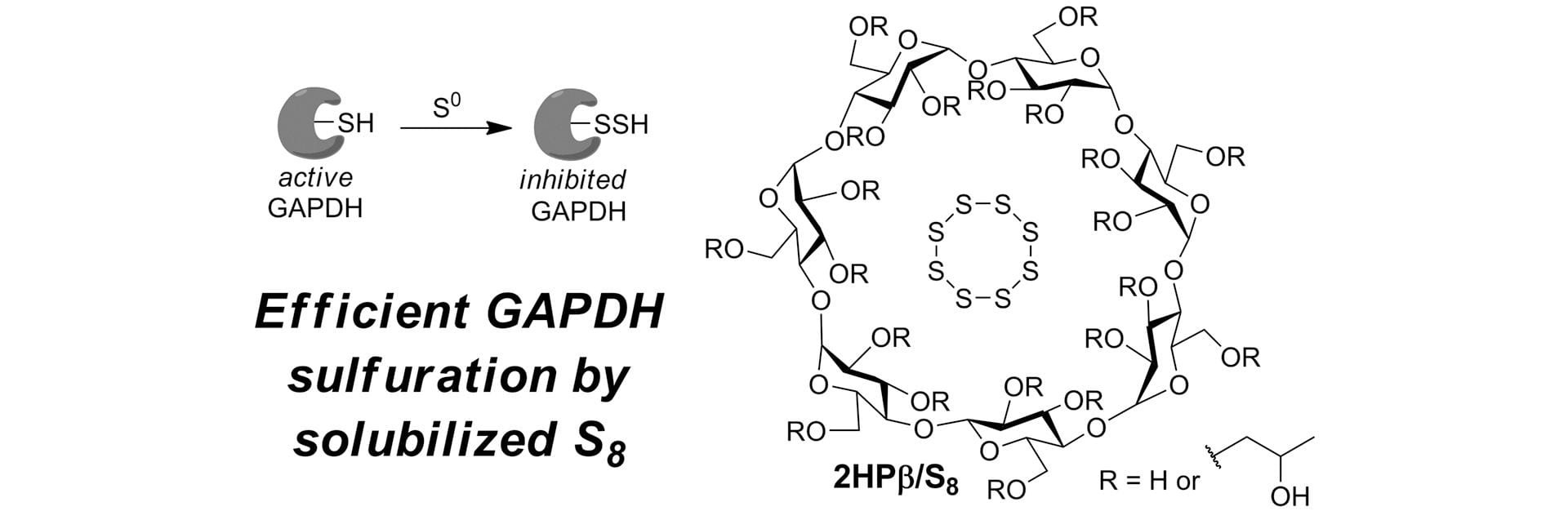
- Bolton, S.G.; Pluth, M.D. “Efficient Inhibition of Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) by Sulfuration with Solubilized Elemental Sulfur.” Free Radic. Biol. Med. 2022, 185, 46-51. [10.1016/j.freeradbiomed.2022.03.032]
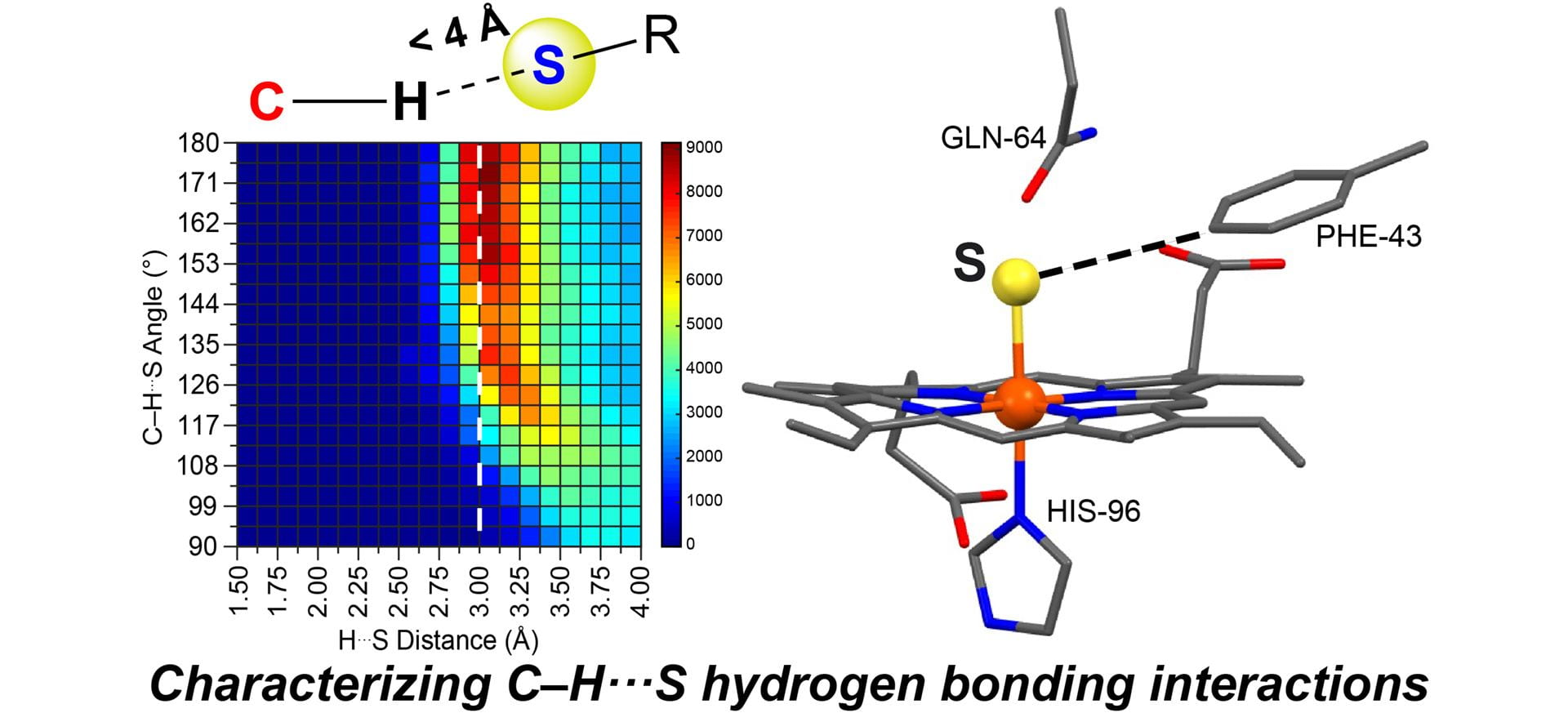
- Fargher, H.A.; Sherbow, T.J.; Haley, M.M.; Johnson, D.W.; Pluth, M.D. “C–H···S Hydrogen Bonding Interactions.” Chem. Soc. Rev. 2022, 51, 1454-1469. [10.1039/D1CS00838B]
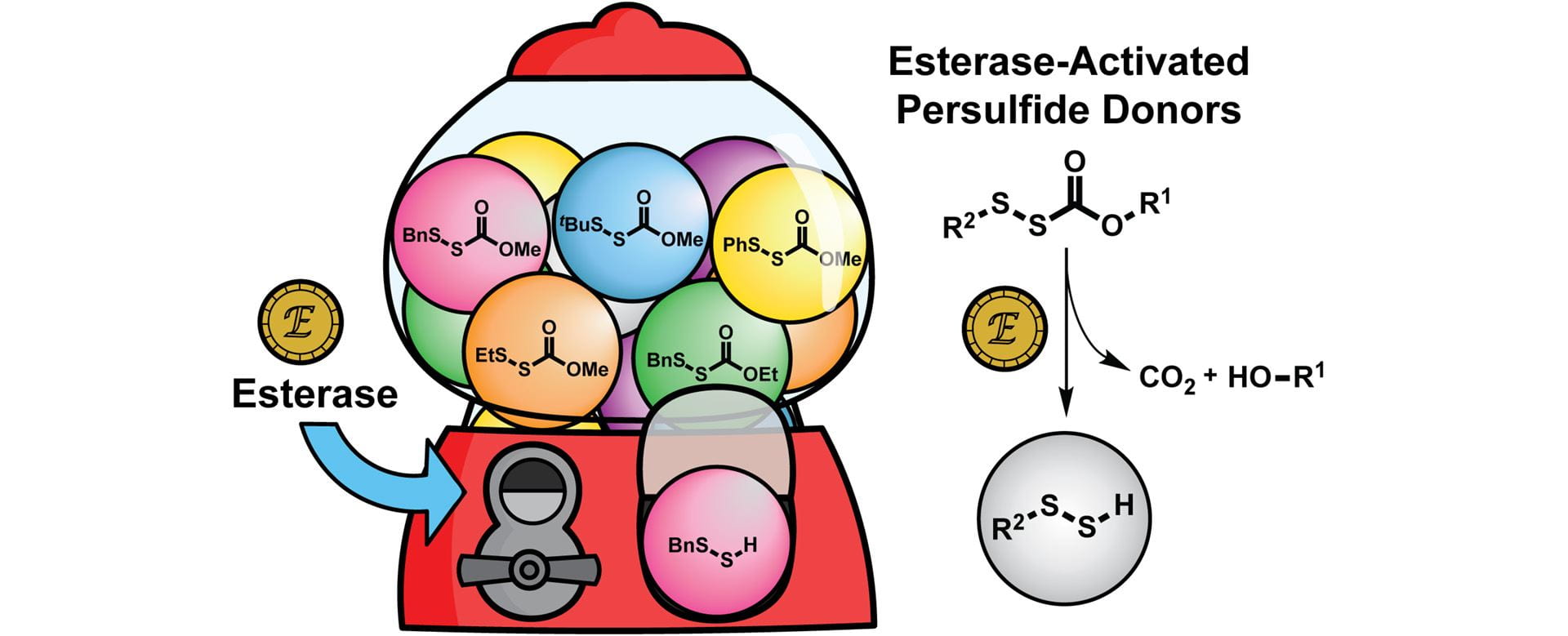
- Fosnacht, K.G.; Cerda, M.M.; Mullen, E.J.; Pigg, H.C.; Pluth, M.D. “Esterase-Activated Perthiocarbonate Persulfide Donors Provide Insights into Persulfide Persistence and Stability.” ACS Chem. Biol. 2022, 17(2), 331-339. [10.1021/acschembio.1c00805]
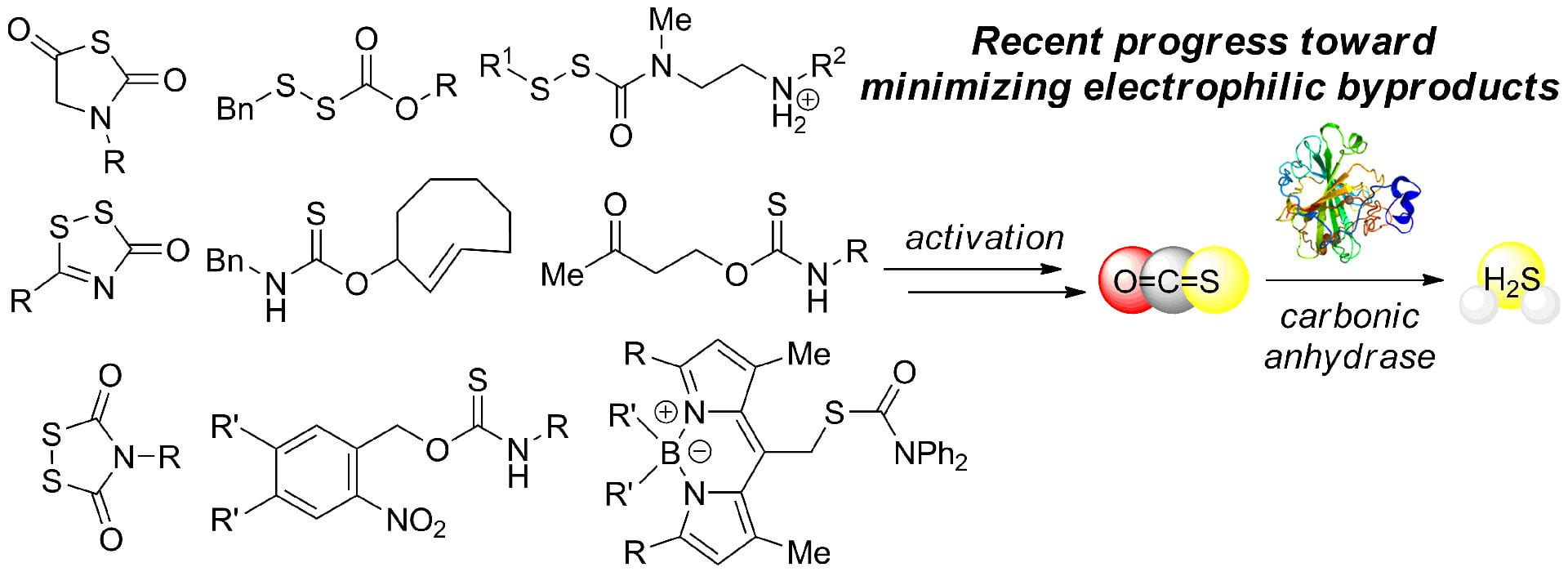
- Pluth, M.D. “Moving Past Quinone-Methides: Recent Advances toward Minimizing Electrophilic Byproducts from COS/H2S Donors.” Curr. Top. Med. Chem. 2021, 21(32), 2882-2889. [10.2174/1568026621666210622130002]
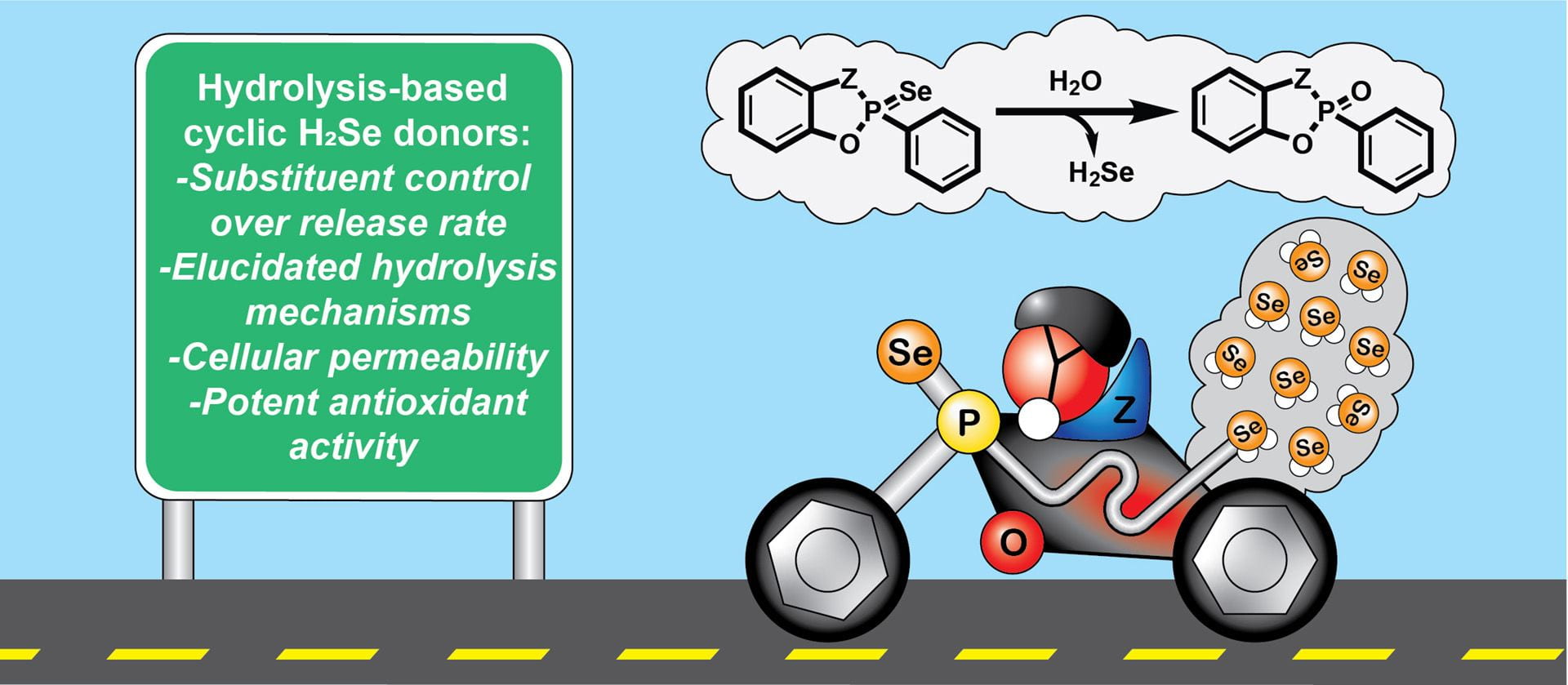
- Newton, T.D.; Bolton, S.G.; Garcia, A.C.; Chouinard, J.E.; Golledge, S.L.; Zakharov, L.N.; Pluth, M.D. “Hydrolysis-Based Small-Molecule Hydrogen Selenide (H2Se) Donors for Intracellular H2Se Delivery.” J. Am. Chem. Soc. 2021, 143(46), 19542–19550. [10.1021/jacs.1c09525]
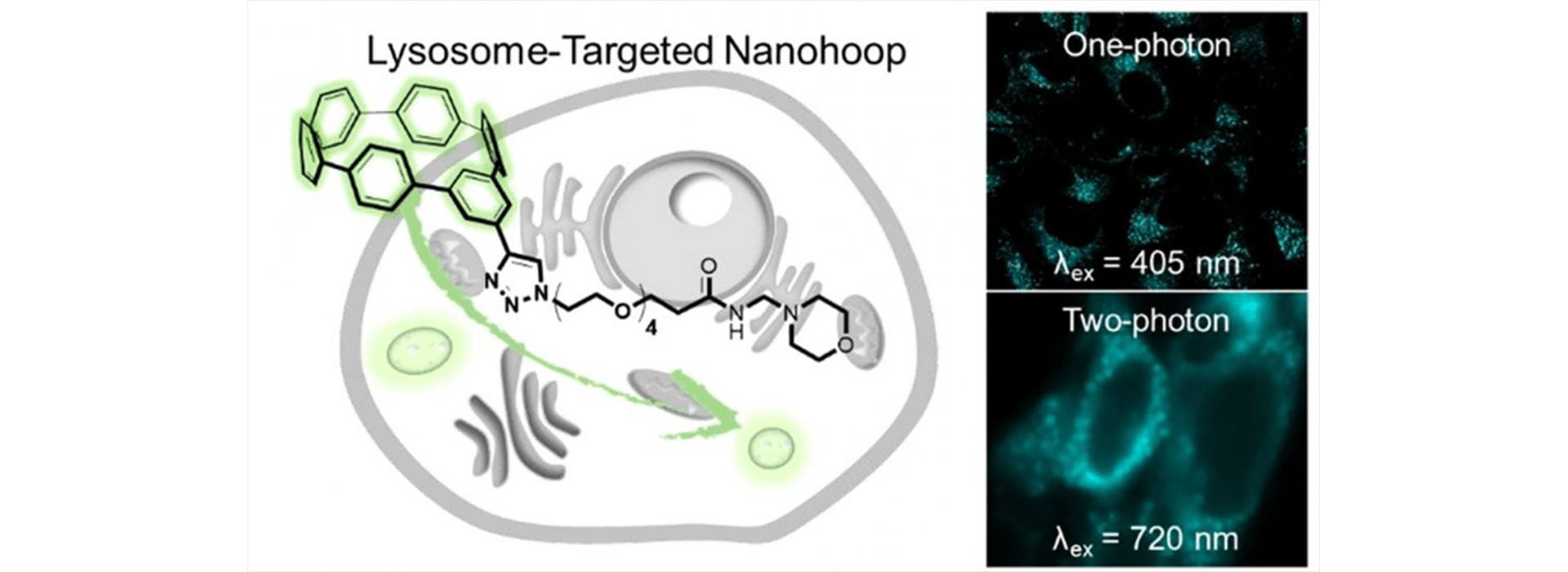
- Lovell, T.C.; Bolton, S.G.; Kenison, J.P.; Shangguan, J.; Otteson, C.E.; Civitci, F.; Nan, X.; Pluth, M.D.; Jasti, R. “Subcellular Targeted Nanohoop for One- and Two-Photon Live Cell Imaging.” ACS Nano 2021, 15(9), 15285–15293 [10.1021/acsnano.1c06070]
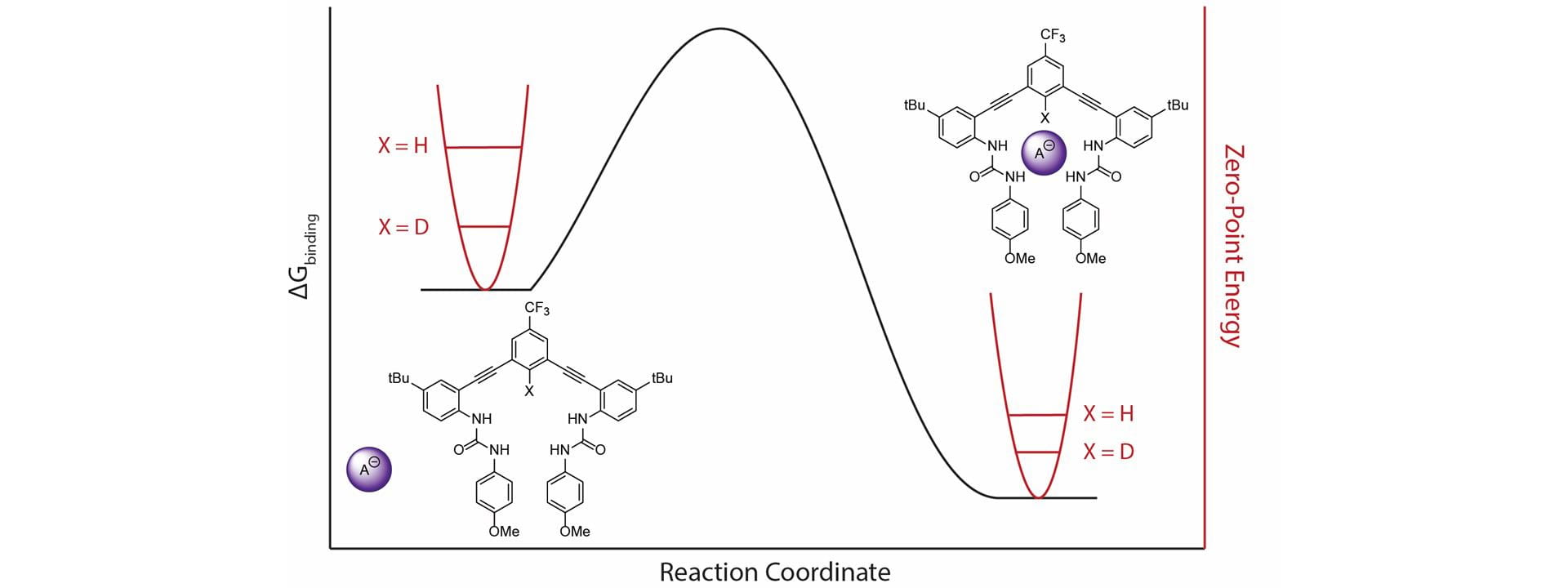
- Fargher, H.A.; Nickels, R.A.; de Faria, T.P.; Haley, M.H.; Pluth, M.D.; Johnson, D.W. “Deuterium Equilibrium Isotope Effects in a Supramolecular Receptor for the Hydrochalcogenide and Halide Anions.” RSC Adv. 2021, 11, 26581-26585 [10.1039/D1RA05711A]
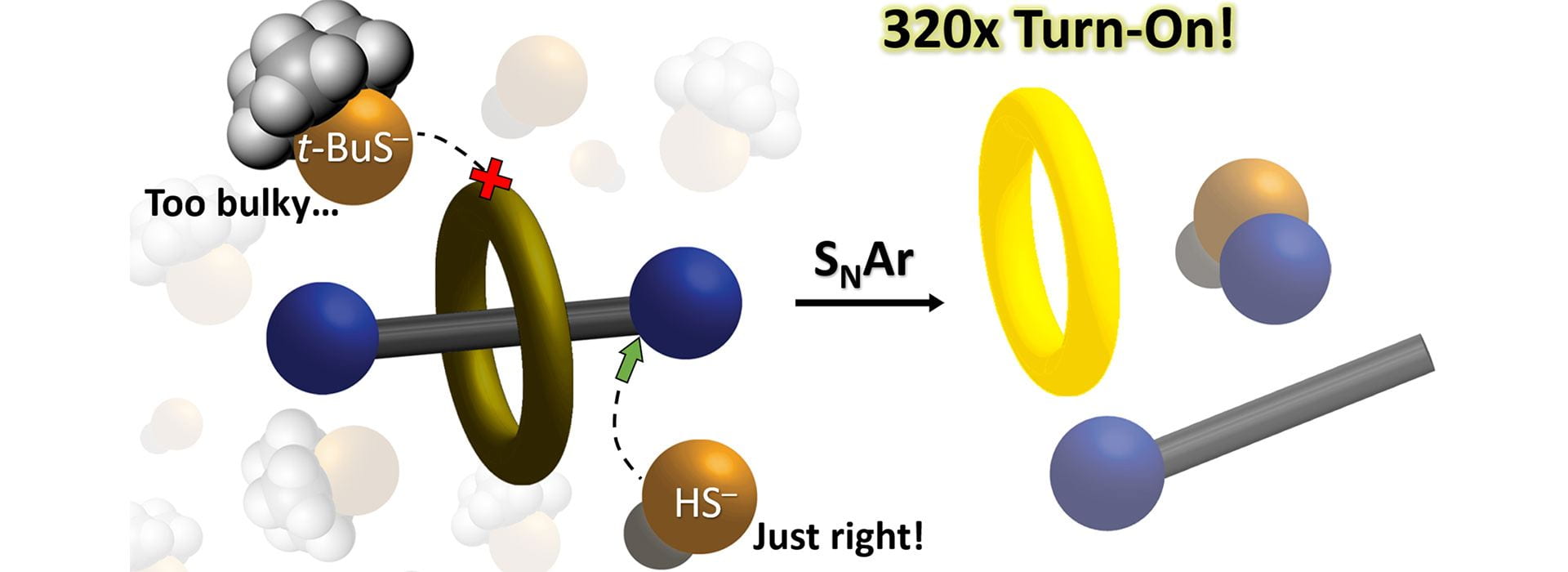
- Otteson, C.E.; Levinn, C.M.; van Raden, J.M.; Pluth, M.D.; Jasti, R. “Nanohoop Rotaxane Design to Enhance Selectivity of Reaction Based Probes: A Proof of Principle Study.” Org. Lett. 2021, 23(12), 4608–4612. [10.1021/acs.orglett.1c01348]
- Jiang, C.; Huang, H.; Kang, X.; Yang, L.; Xi, Z.; Sun, H.; Pluth, M.D.; Yi, L. “NBD-Based Synthetic Probes for Sensing Small Molecules and Proteins: Design, Sensing Mechanisms and Biological Applications.” Chem. Soc. Rev., 2021, 50, 7436-7495. [10.1039/D0CS01096K]
– Inside cover article


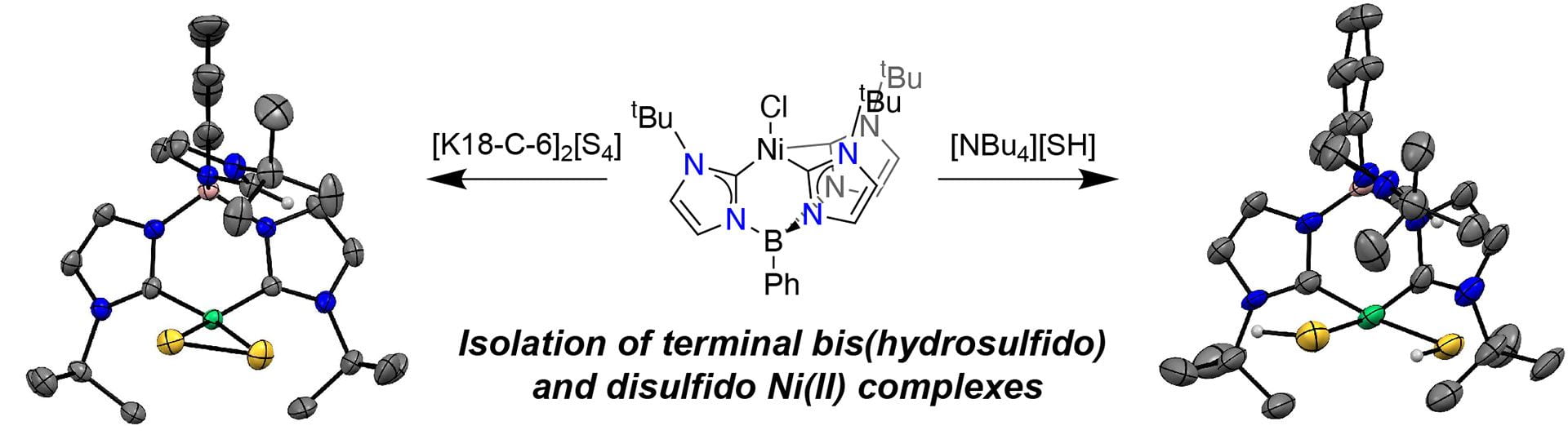
- Sherbow, T.J.; Zakharov, L.N.; Pluth, M.D. “Synthesis of Terminal Bis(hydrosulfido) and Disulfido Complexes of Ni(II) from a Geometrically Frustrated Tetrahedral Ni(II) Chloride Complex.” Inorg. Chem. 2021, 60(11), 8135-8142. [10.1021/acs.inorgchem.1c00787]
- Dóka, É.; Arnér, E.S.J.; Schmidt, E.E.; Dick, T.P.; van der Vliet, A.; Yang, J.; Szatmári, R.; Ditrói, T.; Wallace, J.L.; Cirino, G.; Olson, K.; Motohashi, H.; Fukuto, J.M.; Pluth, M.D.; Feelisch, M.; Akaike, T.; Wink, D.A.; Ignarro, L.J.; Nagy, P. “Comment on ‘Evidence that the ProPerDP method is inadequate for protein persulfidation detection due to lack of specificity.’” Sci. Advances, 2021, 7(17), eabe7006. [10.1126/sciadv.abe7006]
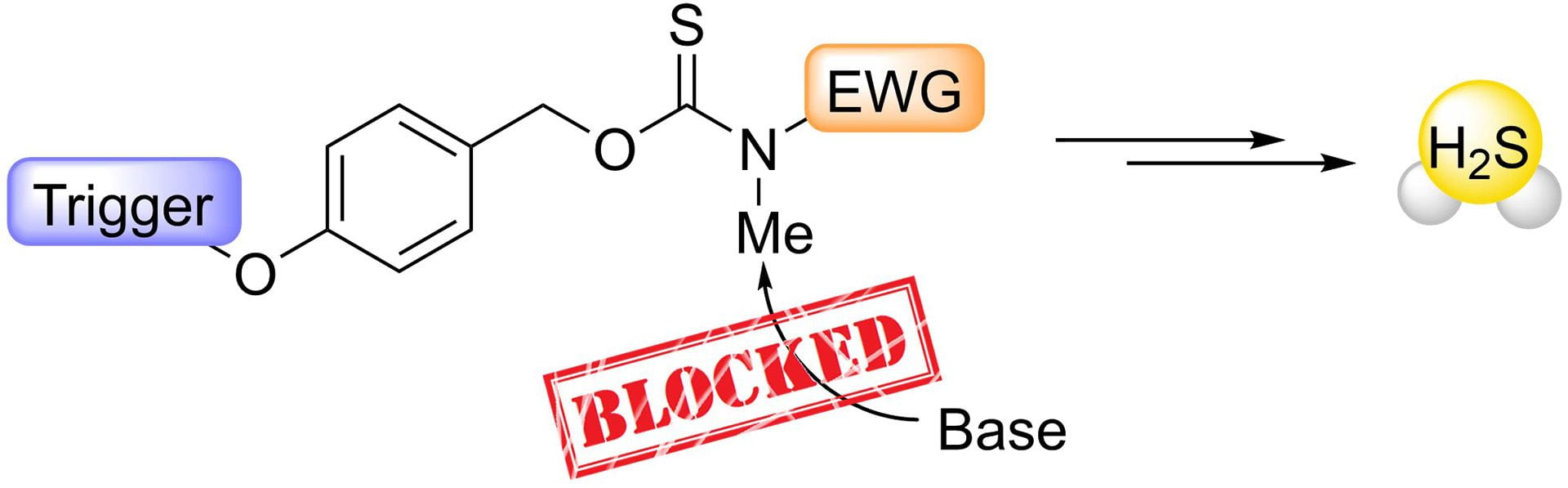
- Levinn, C.M.; Mancuso, J.L; Lutz, R.E.; Smith, H.M.; Hendon, C.H.; Pluth, M.D. “N-Methylation of Self-Immolative Thiocarbamates Provides Insights into the Mechanism of Carbonyl Sulfide Release.” J. Org. Chem., 2021, 86(8), 5443–5451. [10.1021/acs.joc.0c02778]
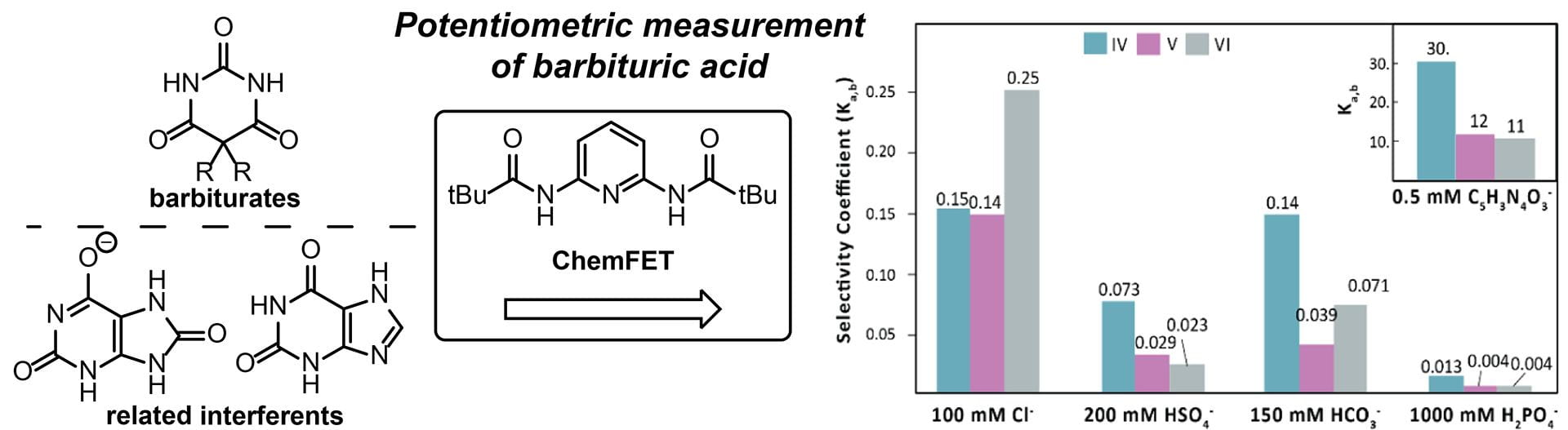
- Kuhl, G.M.; Seidenkranz, D.T.; Pluth, M.D.; Johnson, D.W.; Fontenot, S.A. “Potentiometric Measurement of Barbituric Acid by Integration of Supramolecular Receptors into ChemFETs.” Sens. Bio-Sens. Res., 2021, 100397. [10.1016/j.sbsr.2021.100397]
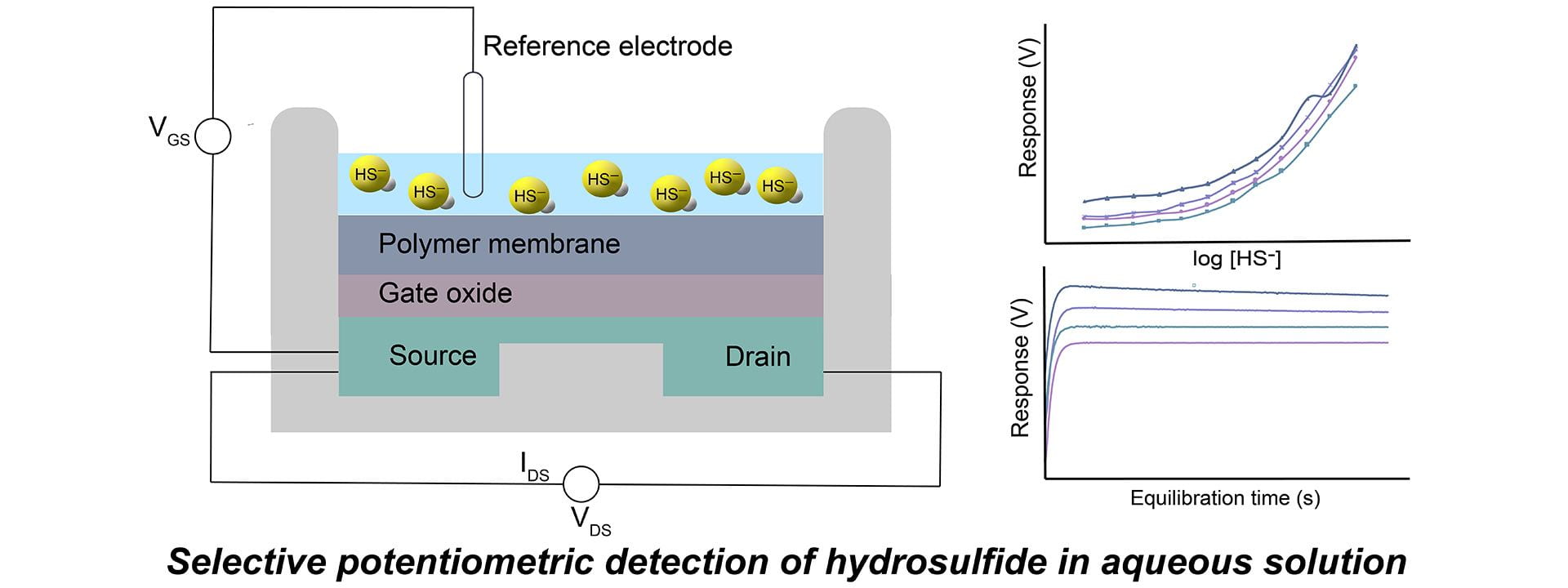
- Sherbow, T.J.; Kuhl, G.M.; Lindquist, G.A.; Levine, J.D.; Pluth, M.D.; Johnson, D.W.; Fontenot, S.A. “Hydrosulfide-Selective ChemFETs for Aqueous H2S/HS− Measurement.” Sens. Bio-Sens. Res., 2021, 100394. [10.1016/j.sbsr.2020.100394]
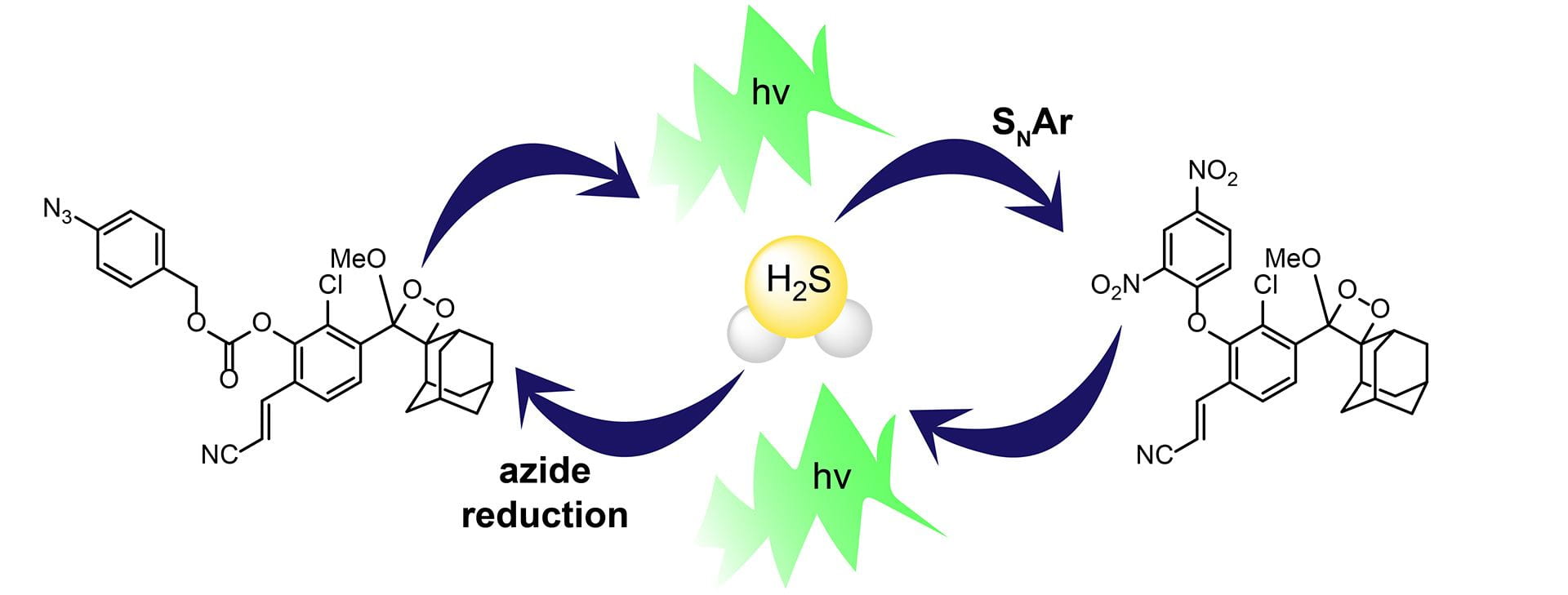
- Levinn, C.M.; Pluth, M.D. “Direct Comparison of Triggering Motifs on Chemiluminescent Probes for Hydrogen Sulfide Detection in Water.” Sens. Actuators B Chem., 2021, 329(15), 129235. [10.1016/j.snb.2020.129235]
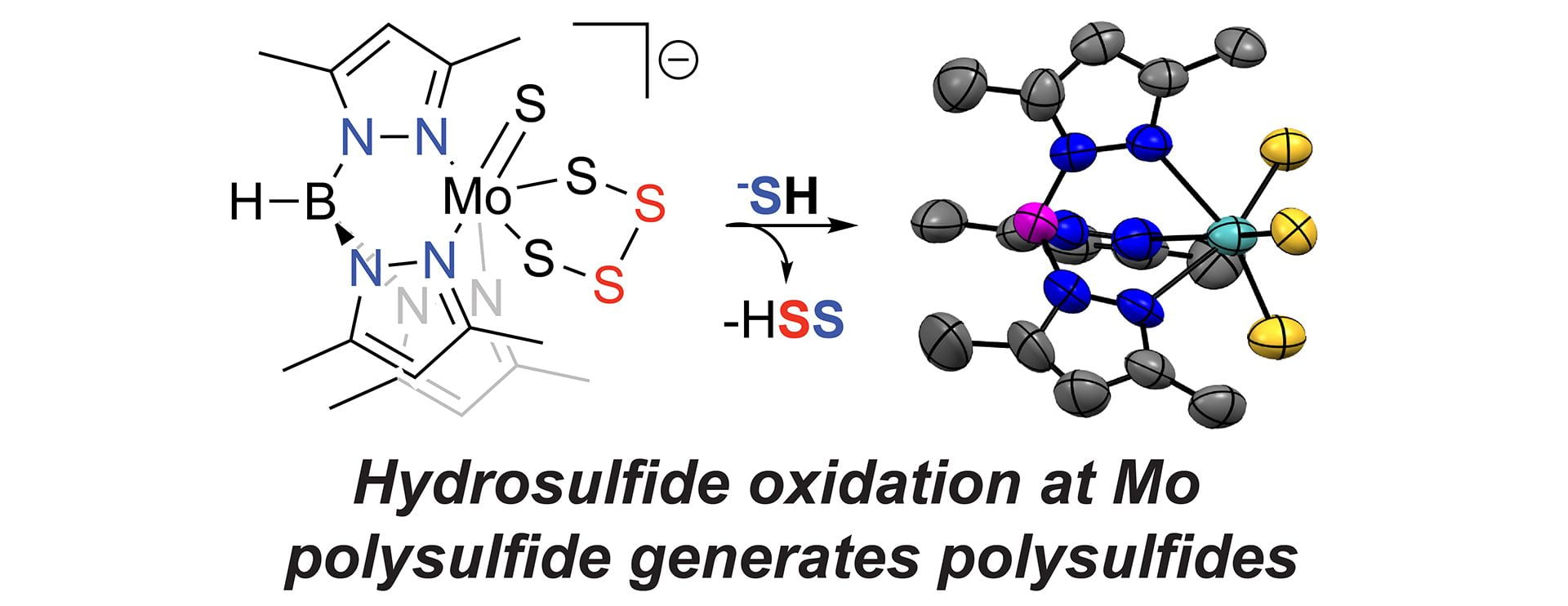
- Sherbow, T.J.; Zakharov, L.N.; Johnson, D.W.; Pluth, M.D. “Hydrosulfide Oxidation at a Molybdenum-Tetrasulfido Complex.” Inorg. Chem. 2020, 59(21), 15574–15578. [10.1021/acs.inorgchem.0c02622]
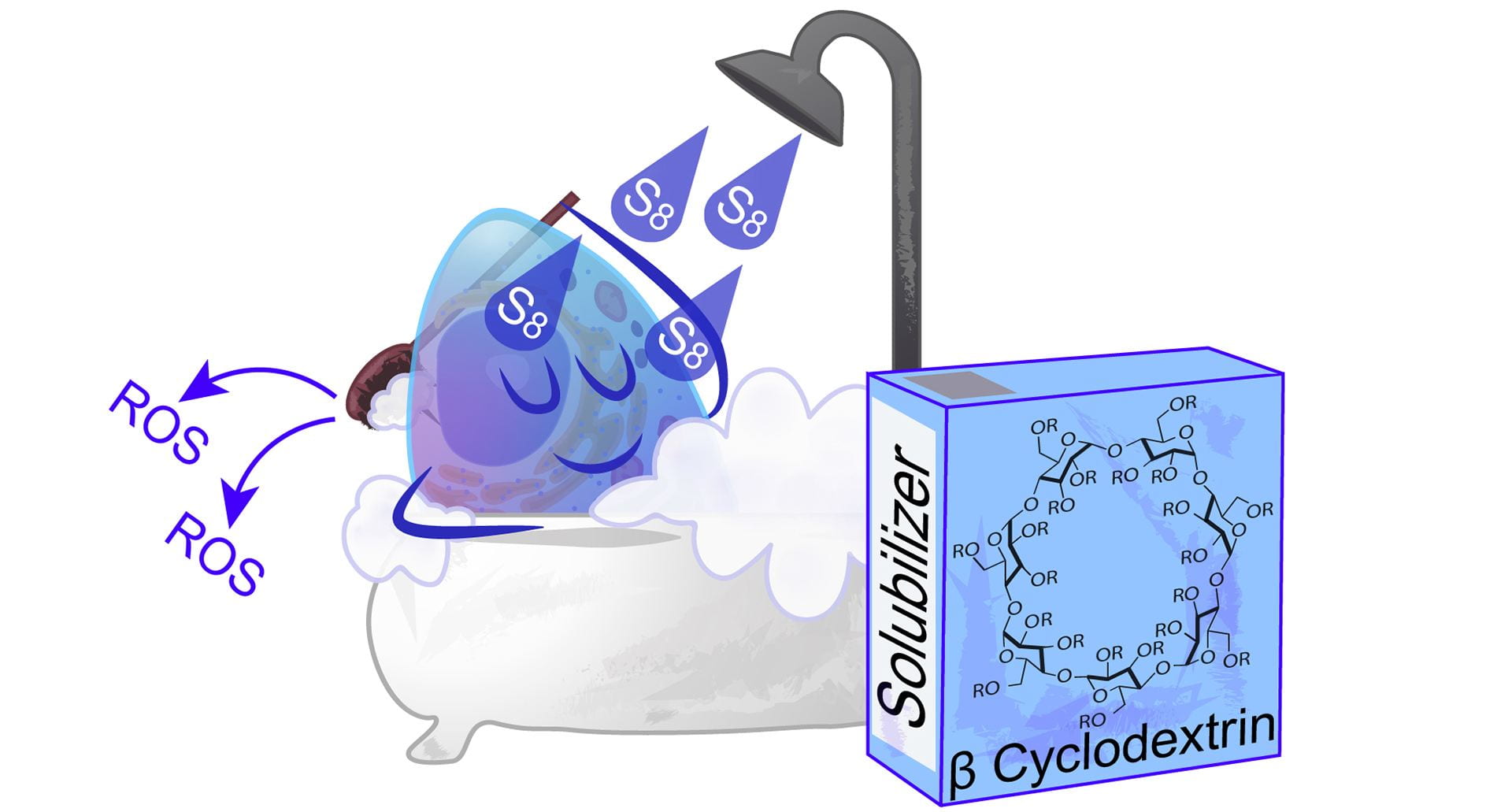
- Bolton, S.G.; Pluth, M.D. “Modified Cyclodextrins Solubilize Elemental Sulfur in Water and Enable Biological Sulfane Sulfur Delivery.” Chem. Sci. 2020, 11, 11777-11784. [10.1039/D0SC04137H]
- Sherbow, T.J.; Fargher, H.A.; Haley, M.M.; Pluth, M.D. Johnson, D.W. “Solvent-Dependent Linear Free Energy Relationship in a Flexible Host-Guest System.” J. Org. Chem. 2020, 85(19), 12367–12373. [110.1021/acs.joc.0c01616]
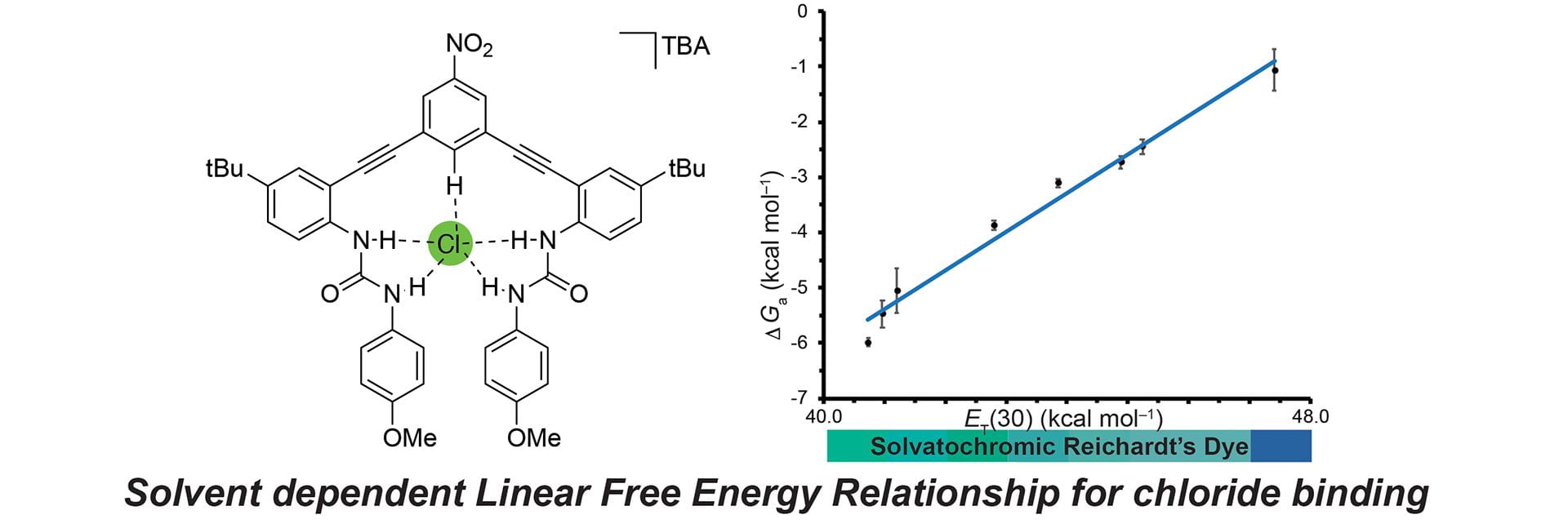
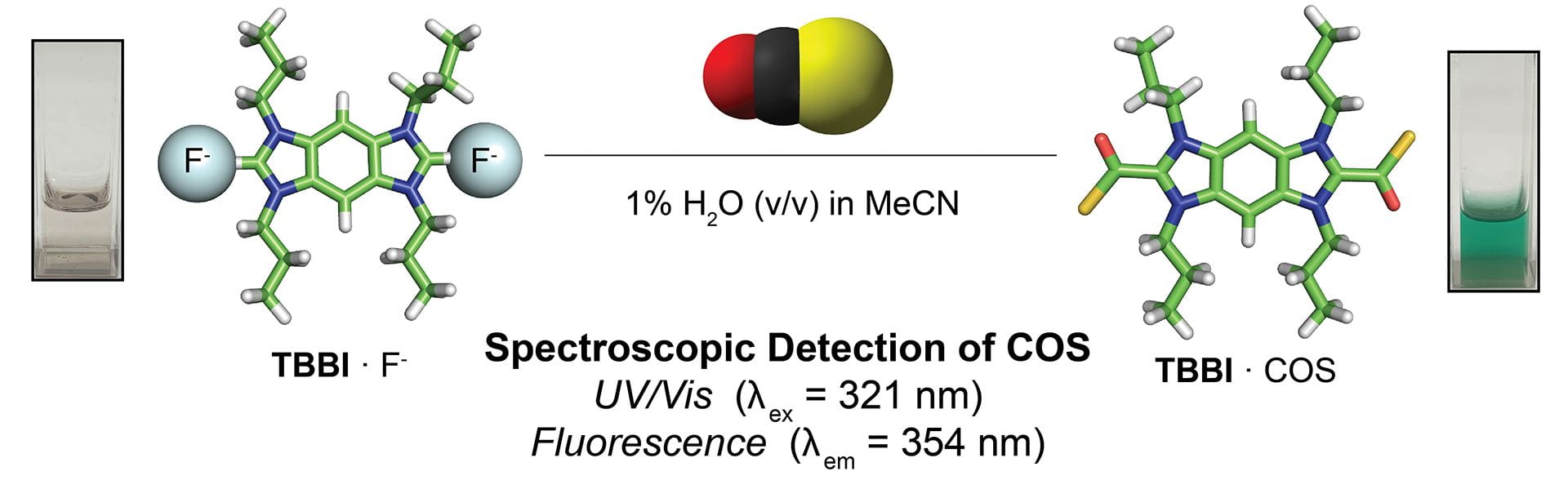
- Cerda, M.M.; Fehr, J.M.; Sherbow, T.J.; Pluth, M.D “Progress Toward Colorimetric and Fluorescent Detection of Carbonyl Sulfide.” Chem. Commun. 2020, 56, 9644-9647. [10.1039/D0CC04528D ]
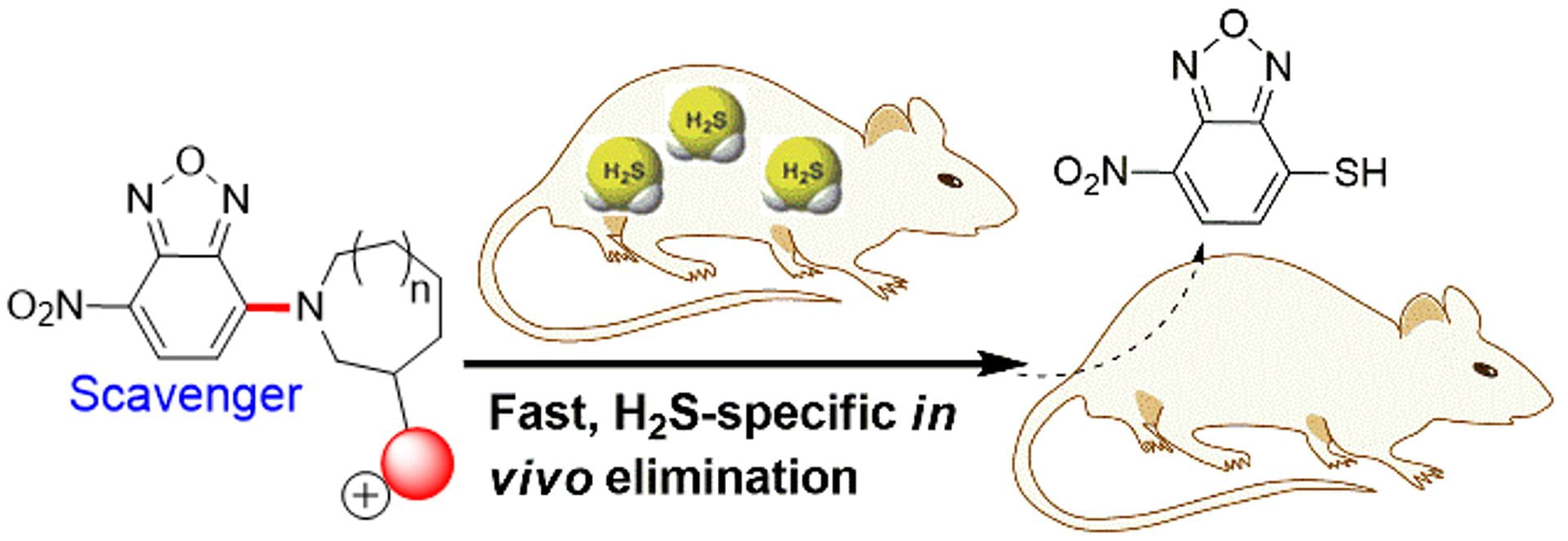
- Ismail, I.; Chen, Z.; Sun, L.; Ji, X.; Ye, H.; Kang, X.; Huang, H.; Song, H.; Bolton, S.G.; Xi, Z.; Pluth, M.D.; Long, Y. “Highly Efficient H2S Scavengers via Thiolysis of Positively-Charged NBD Amines” Chem. Sci. 2020, 11, 7823-7828. [10.1039/D0SC01518K]
- Pluth, M.D.; Zhao, Y.; Cerda, M.M. “H2S Donors with Optical Responses.” Method. Enzymol. 2020, 641, 149-164. [10.1016/bs.mie.2020.04.039]
- Pluth, M.D.; Tonzetich, Z.J. “Hydrosulfide Complexes of the Transition Elements: Diverse Roles in Bioinorganic, Cluster, Coordination, and Organometallic Chemistry.” Chem. Soc. Rev. 2020, 49, 4070-4134. [10.1039/c9cs00570f]
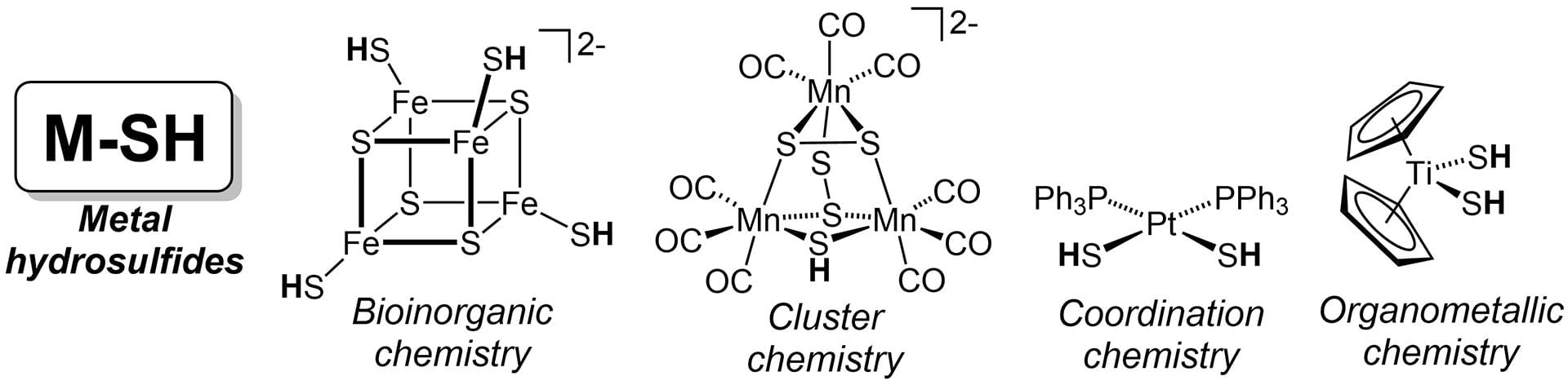
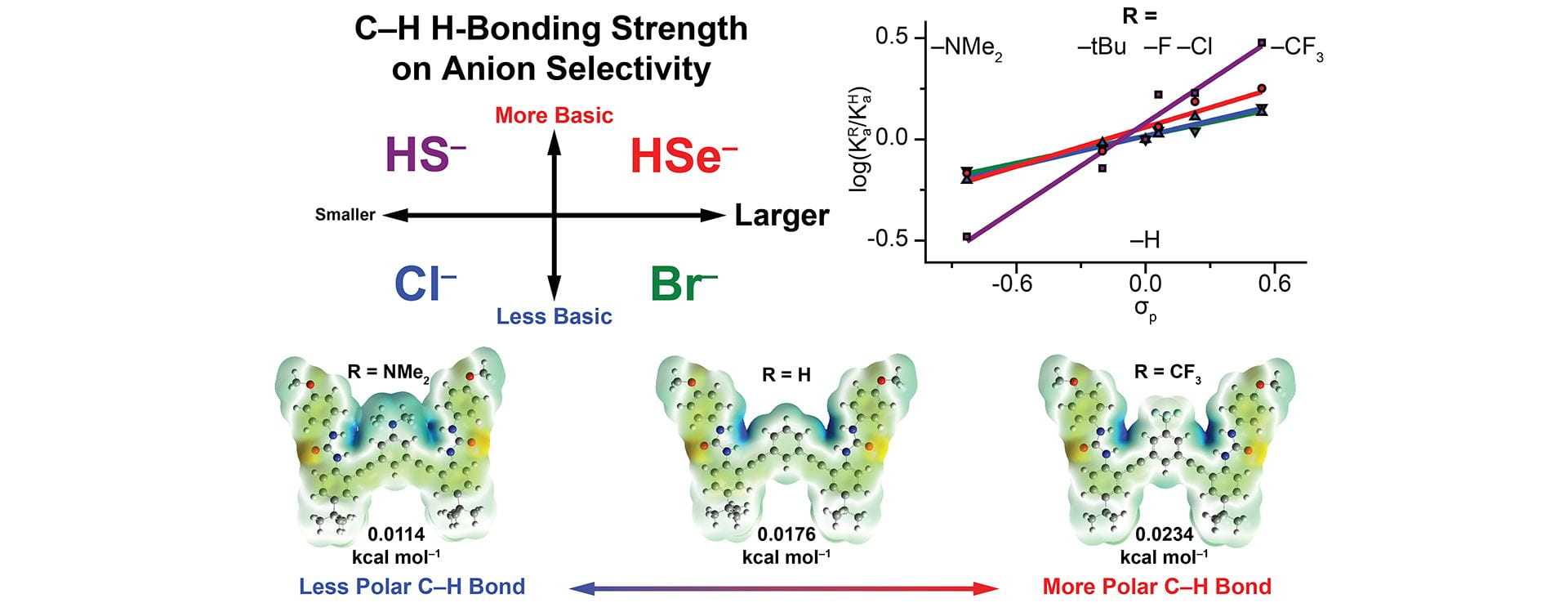
- Fargher, H.A.; Lau, N.; Richardson, H.C.; Cheong, P.H-Y.; Haley, M.M.; Pluth, M.D.; Johnson, D.W. “Tuning Supramolecular Selectivity for Hydrosulfide: Linear Free Energy Relationships Reveal Preferential C–H Hydrogen Bond Interactions.” J. Am. Chem. Soc. 2020, 142(18), 8243–8251. [10.1021/jacs.0c00441]
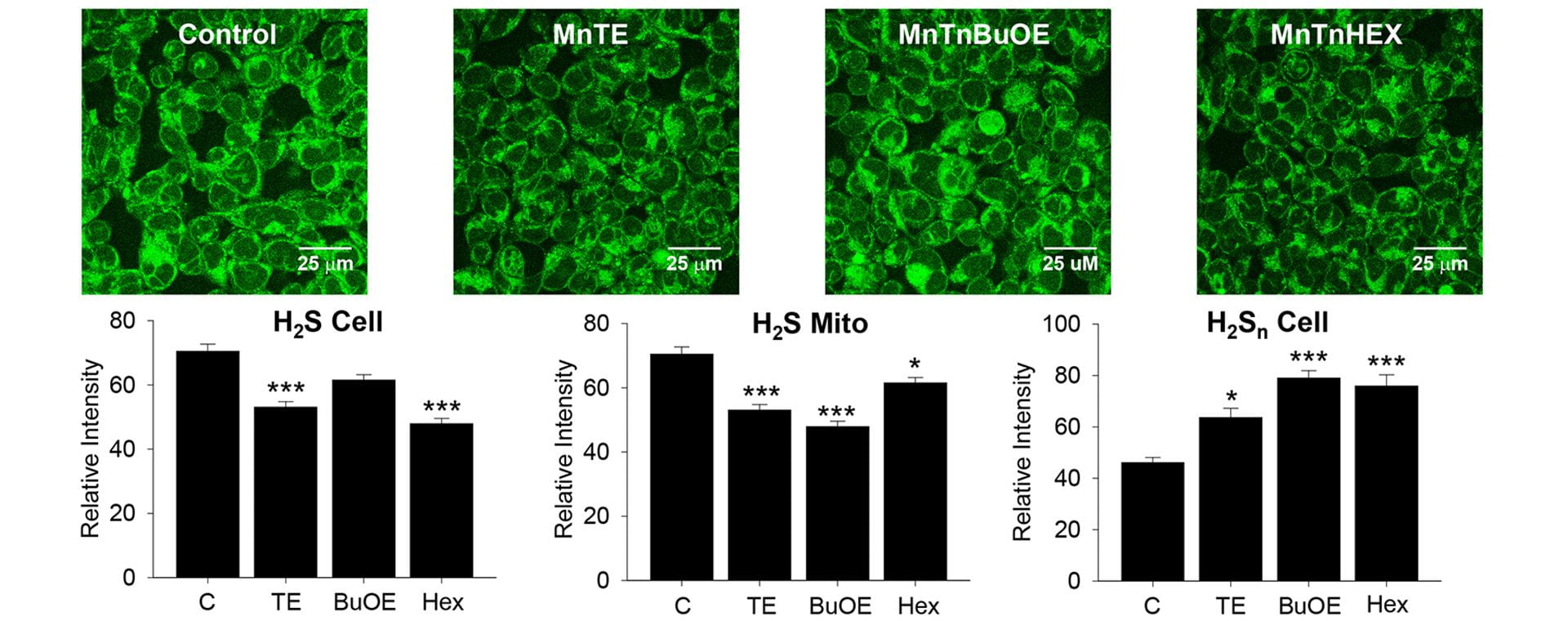
- Olson, K.R.; Gao, Y.; Steiger, A.K.; Pluth, M.D.; Tessier, C.R.; Markel, T.A.; Boone, D.; Stahelin, R.V.; Batinic-Haberle, I.; Straubg, K.D. “Effects of Manganese Porphyrins on Cellular Sulfur Metabolism.” Molecules. 2020, 25(4), 980. [10.3390/molecules25040980]
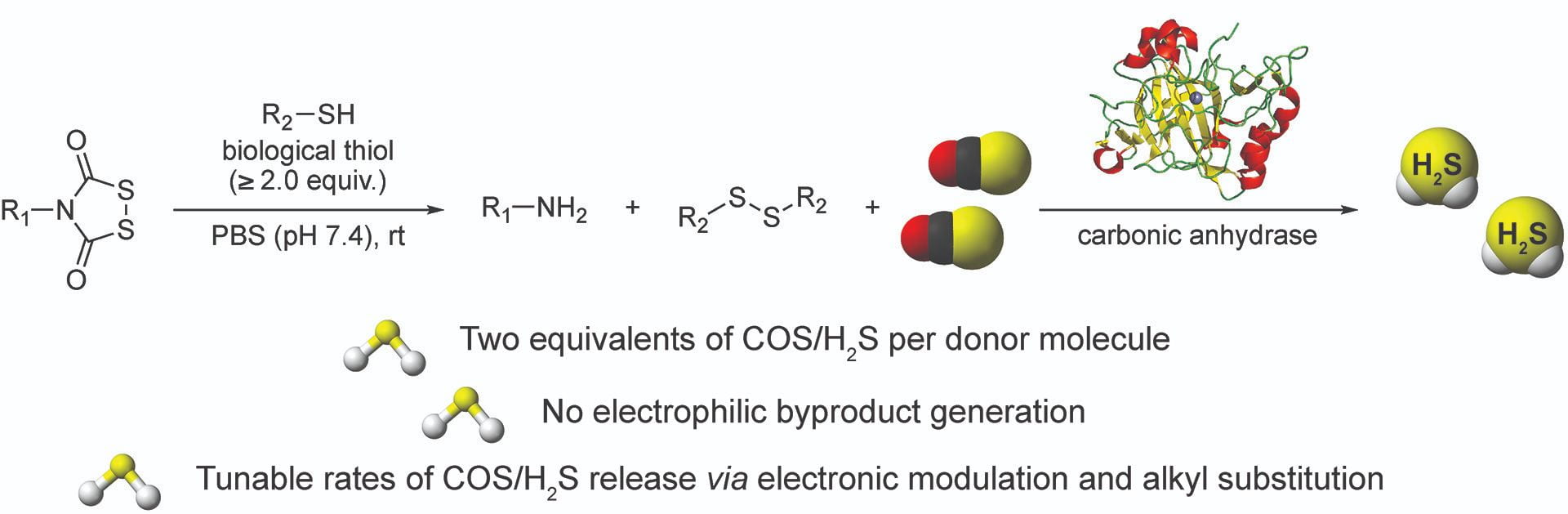
- Cerda, M.M.; Mancuso, J.L.; Mullen, E.J.; Hendon, C.H.; Pluth, M.D. “Dithiasuccinoyl-Caged Amines Enables COS/H2S Release Lacking Electrophilic Byproducts” Chem. Eur. J. 2020, 26(24), 5374-5380. [10.1002/chem.201905577]
- Levinn, C.M; Cerda, M.M.; Pluth, M.D. “Activatable Small Molecule H2S Donors” Antioxid. Redox Signal. 2020, 32(2), 96-109. [10.1089/ars.2019.7841]
– Supporting Information: Mechanisms of H2S release from donors covered in the review.
- Newton, T.D.; Pluth, M.D. “Development of a Hydrolysis-Based Small-Molecule Hydrogen Selenide (H2Se) Donor” Chem. Sci. 2019, 10, 10723-10727. [10.1039/C9SC04616J]
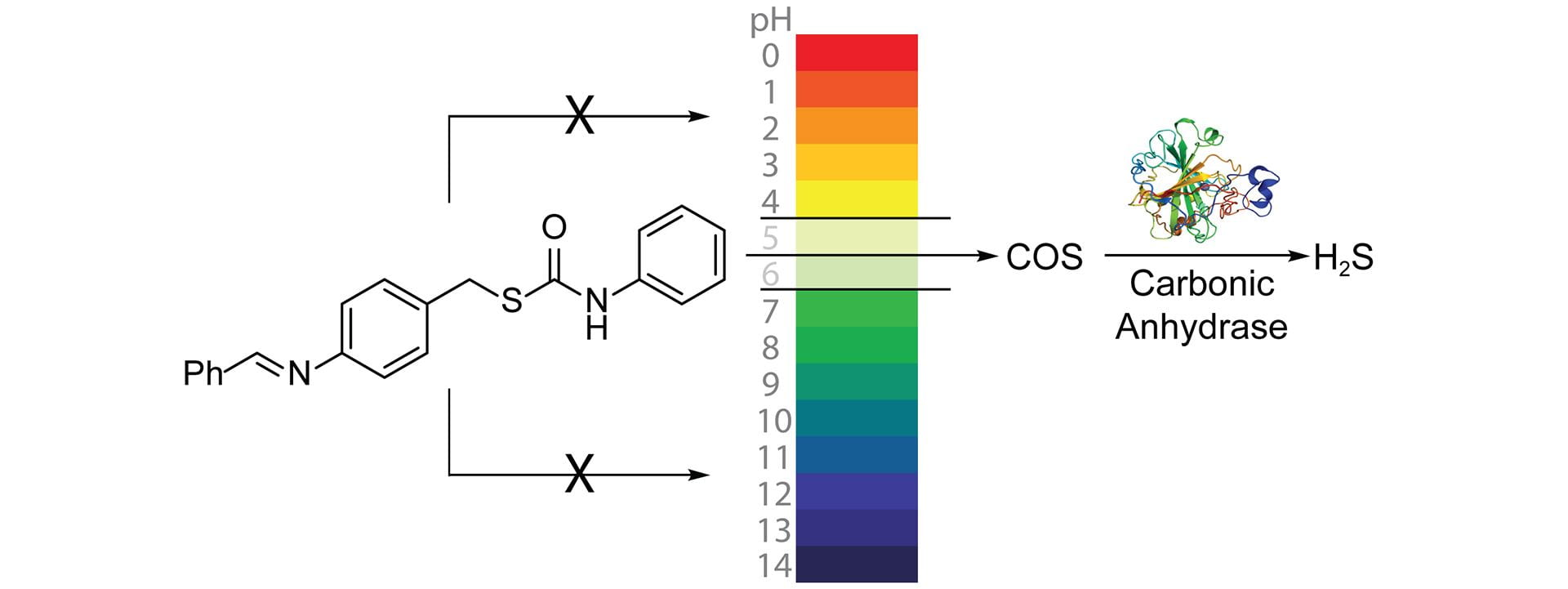
- Gilbert, A.K.; Zhao, Y.; Otteson, C.E.; Pluth, M.D. “Development of Acid-Mediated H2S/COS Donors that Respond to a Specific pH Window.” J. Org. Chem. 2019, 84(22), 14469-14475. [10.1021/acs.joc.9b01873]
– Cover article

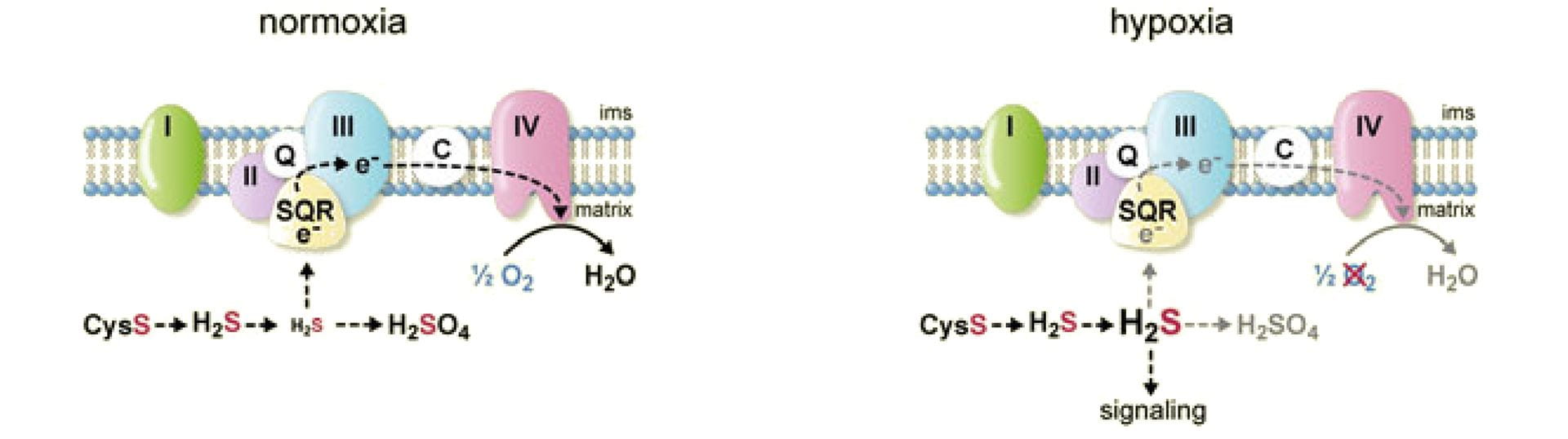
- Olson, K.R.; Gao, Y.; DeLeon, E.R.; Markel, T.A.; Drucker, N.; Boone, D.; Whiteman, M.; Steiger, A.K.; Pluth, M.D.; Tessier, C.R.; Stahelin, R.V. “Extended Hypoxia-Mediated H2S Production Provides for Long-Term Oxygen Sensing.” Acta Physiol. 2019, 228(3), e13368. [10.1111/apha.13368]
- Zhao, Y.; Steiger, A.K.; Pluth, M.D. “Cyclic Sulfenyl Thiocarbamates Release Carbonyl Sulfide (COS) and Hydrogen Sulfide (H2S) Independently in Thiol-Promoted Pathways.” J. Am. Chem. Soc. 2019, 41(34), 13610-13618. [10.1021/jacs.9b06319]
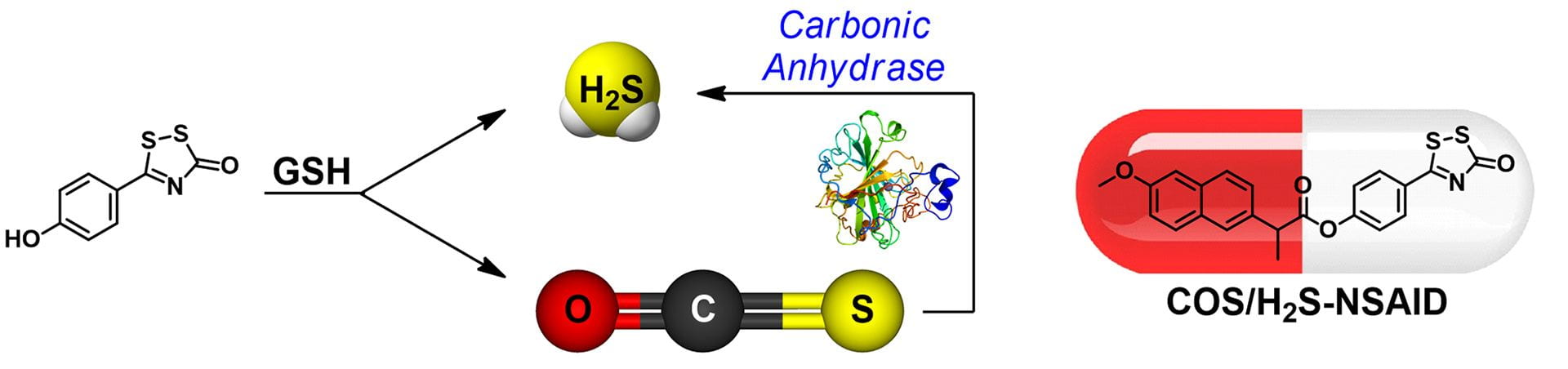
- Levinn, C.M.; Cerda, M.M.; Pluth, M.D. “Development and Application of Carbonyl Sulfide-Based Donors for H2S Delivery.” Acc. Chem. Res. 2019, 52(9), 2723-2731. [10.1021/acs.accounts.9b00315]
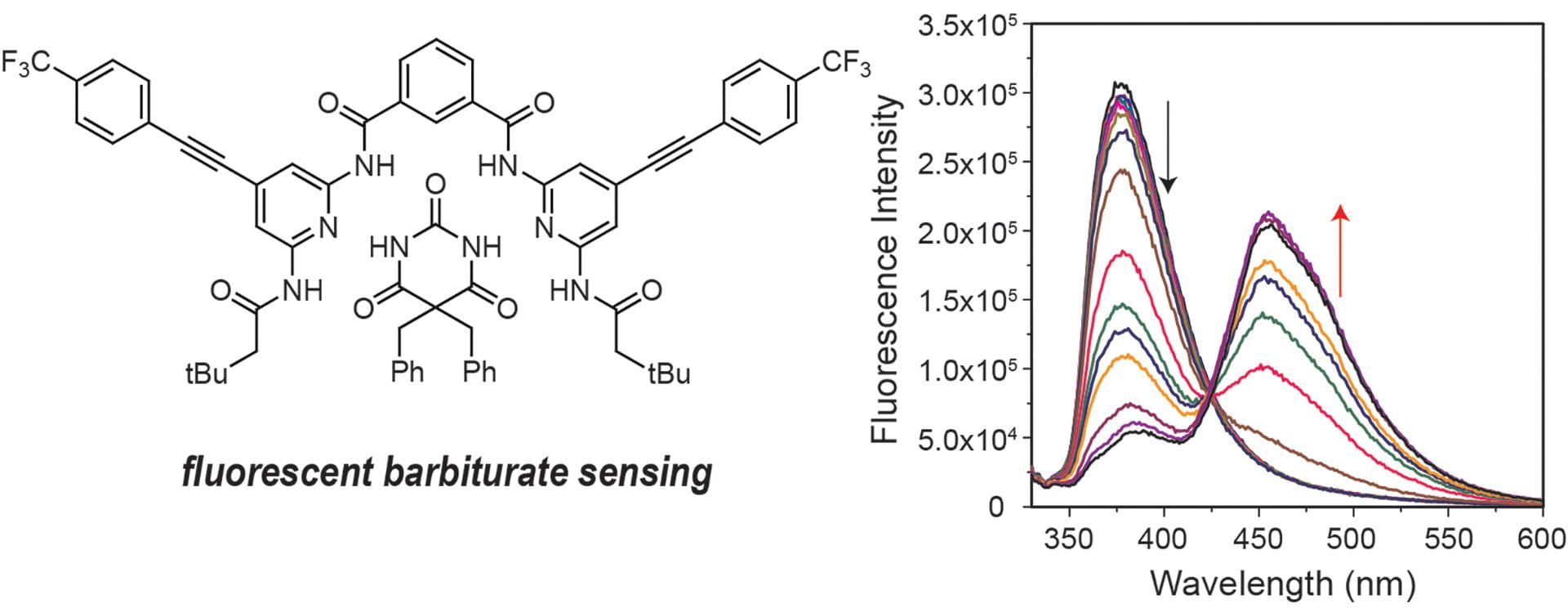
- Seidenkranz, D.T.; Pluth, M.D. “Fluorescent Arylethynyl Hamilton Receptors for Barbiturate Sensing.” J. Org. Chem. 2019, 84(13), 8571-8577. [10.1021/acs.joc.9b00978]
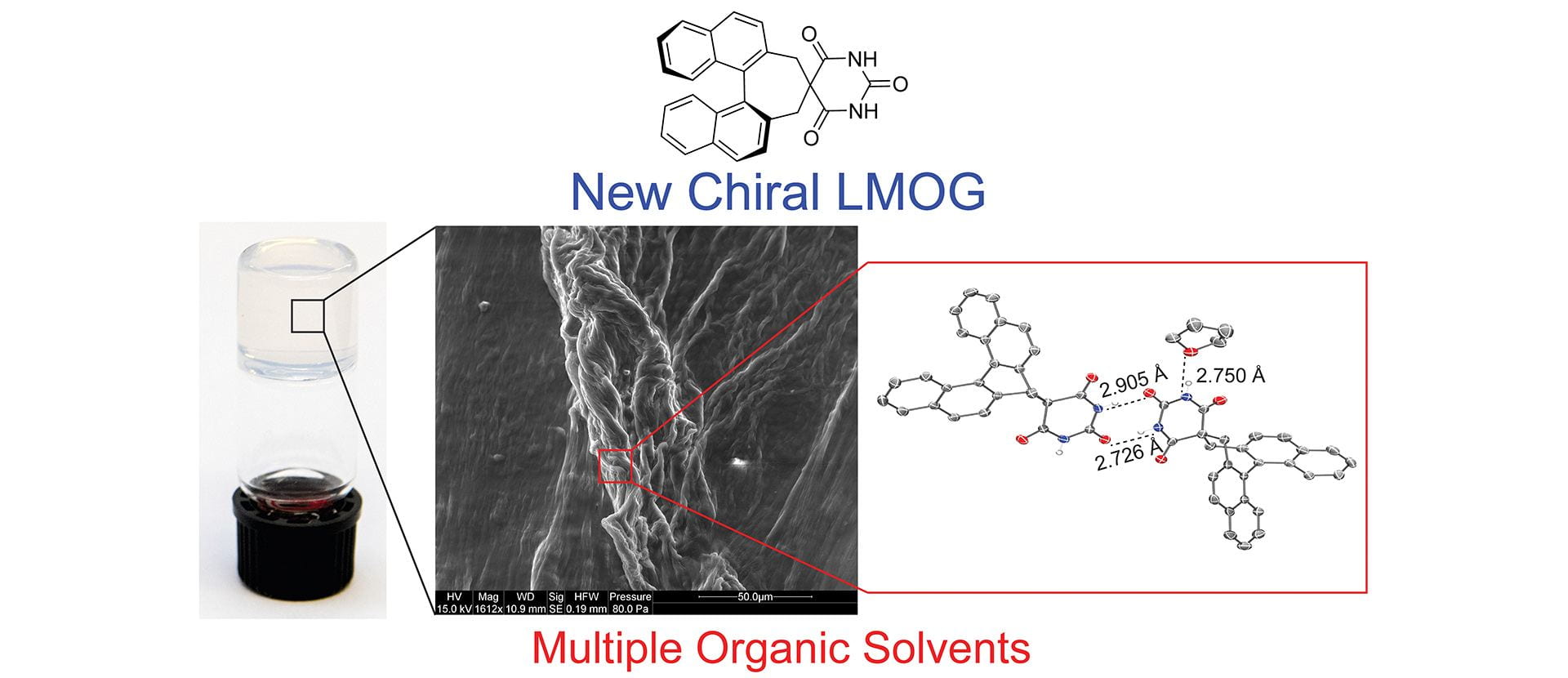
- Seidenkranz, D.T.; Langworthy, K.A.; Zakharov, L.N.; Pluth, M.D. “Single-Component, Low Molecular Weight Organic Supergelators Based on Chiral Barbiturate Scaffolds.” Supramol. Chem. 2019, 31(8), 499-507. [10.1080/10610278.2019.1629437]
- Levinn, C.M; Steiger, A.K. Pluth, M.D. “Esterase-Triggered Self-Immolative Thiocarbamates Provide Insights into COS Cytotoxicity.” ACS Chem. Biol. 2019, 14(2), 170-175. [10.1021/acschembio.8b00981]
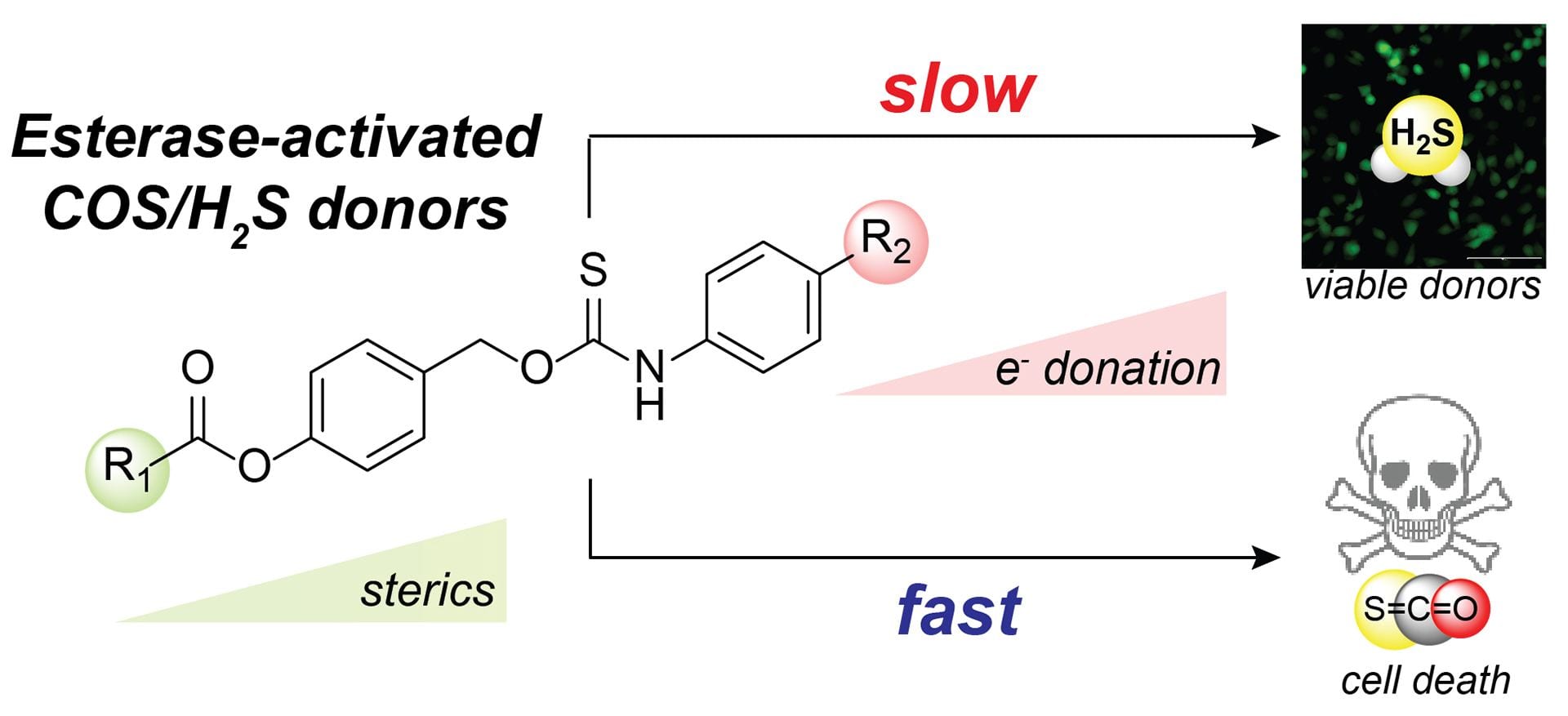
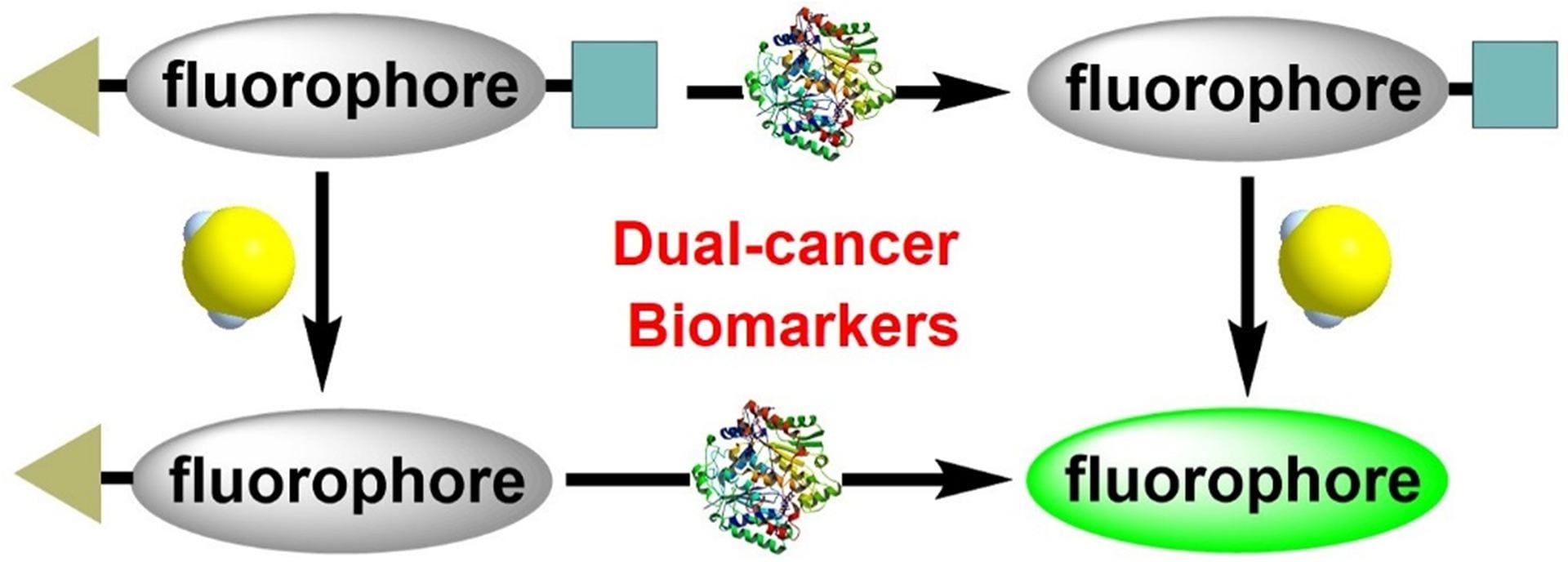
- Zhang, C.; Zhange. Q.Z.; Zhang, K.; Li, L.Y.; Pluth, M.D.; Yi, L. Xi, Z. “Dual-Biomarker-Triggered Fluorescence Probes for Differentiating Cancer Cells and Revealing Synergistic Antioxidant Effects Under Oxidative Stress.” Chem. Sci. 2019, 10, 1945-1952. [10.1039/C8SC03781G]
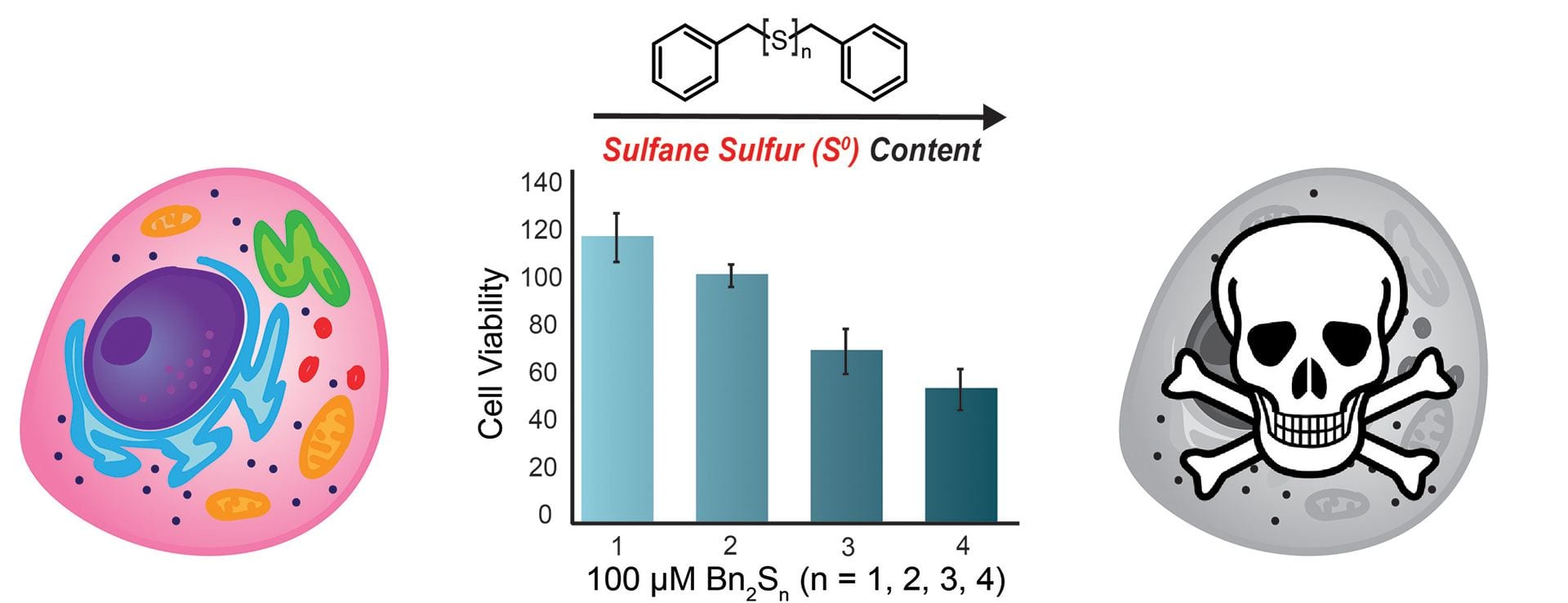
- Bolton, S.G.; Cerda, M.M; Gilbert, A.K.; Pluth, M.D. “Effects of Sulfane Sulfur Content in Benzyl Polysulfides on Thiol-Triggered H2S Release and Cell Proliferation.” Free Radic. Biol. Med. 2019, 131, 393-398. [10.1016/j.freeradbiomed.2018.12.025]
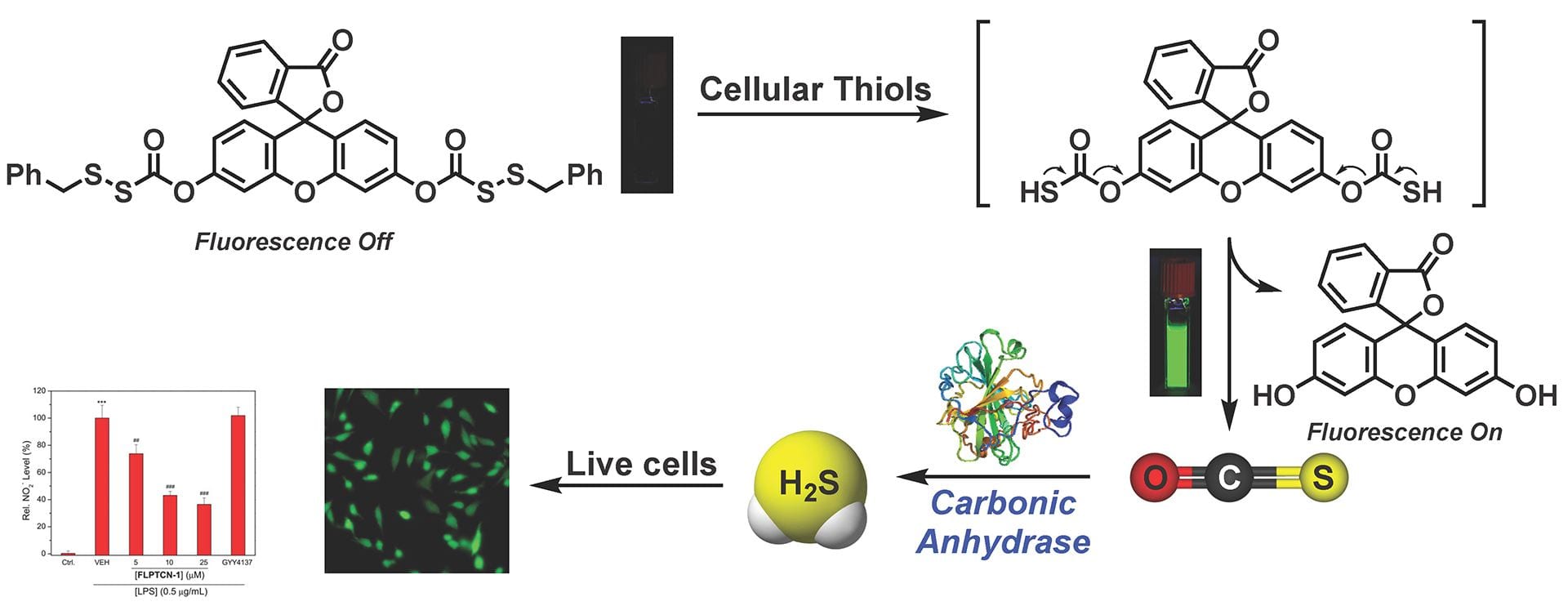
- Zhao, Y.; Cerda, M.M; Pluth, M.D. “Fluorogenic Hydrogen Sulfide (H2S) Donors Based on Sulfenyl Thiocarbonates Enable H2S Tracking and Quantification ” Chem. Sci. 2019,10, 1873-1878. [10.1039/C8SC05200J]
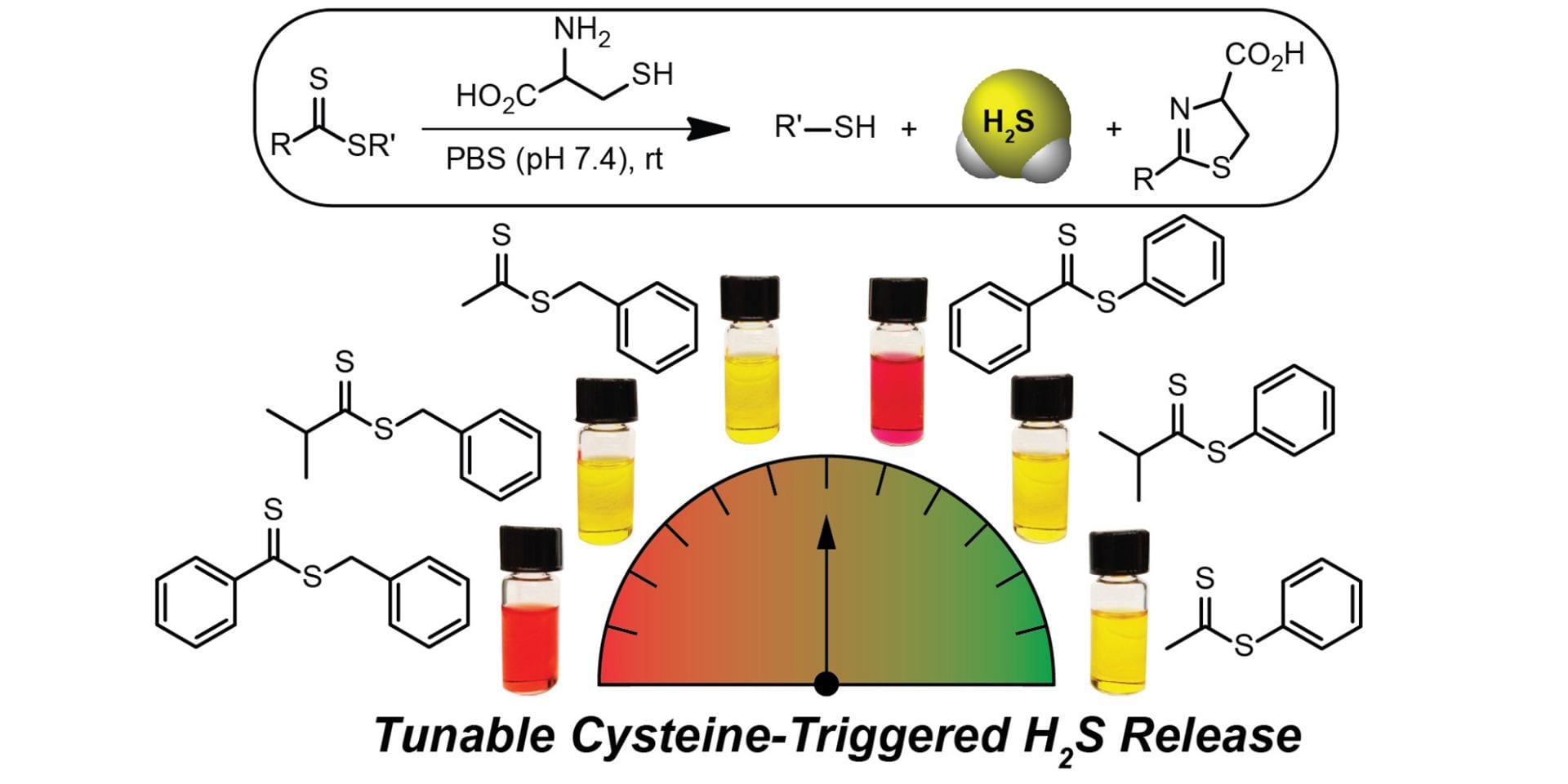
- Cerda, M.M; Newton, T.D.; Zhao, Y.; Collins, B.K.; Hendon, C.H.; Pluth, M.D. “Dithioesters: Simple, Tunable, Cysteine-Selective H2S Donors” Chem. Sci. 2019, 10, 1773-1779. [10.1039/C8SC04683B]
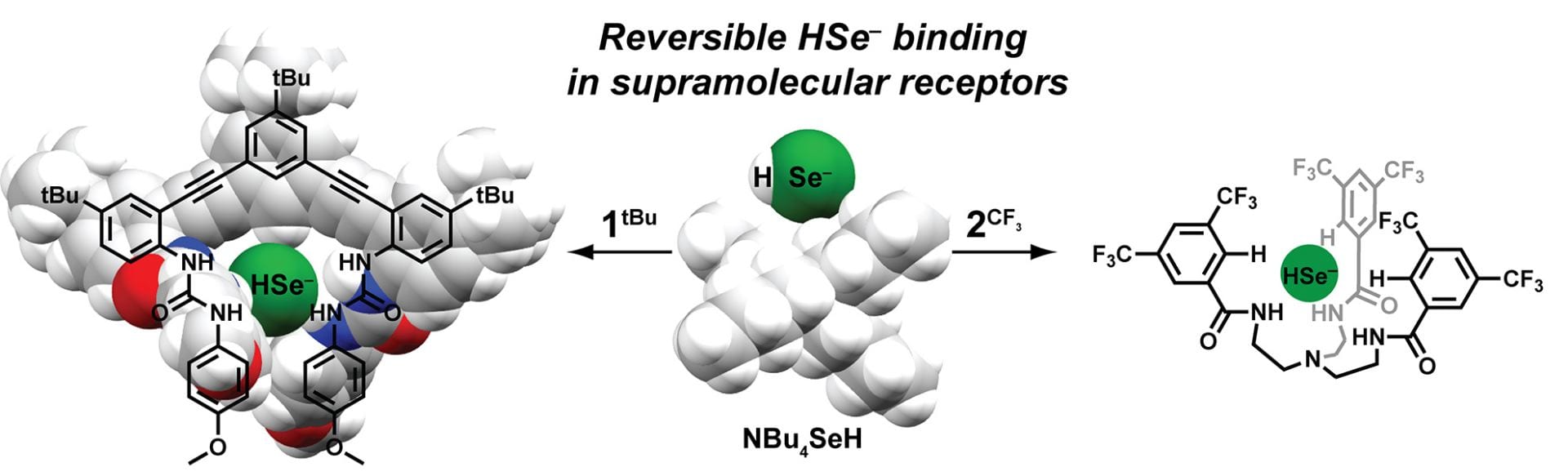
- Fargher, H.A.; Lau, N.; Zakharov, L.N.; Haley, M.M.; Johnson, D.W.; Pluth, M.D. “Expanding Reversible Chalcogenide Binding: Supramolecular Receptors for the Hydroselenide (HSe–) Anion” Chem. Sci. 2019, 10, 67-72. [10.1039/C8SC03968B]
– Back Cover

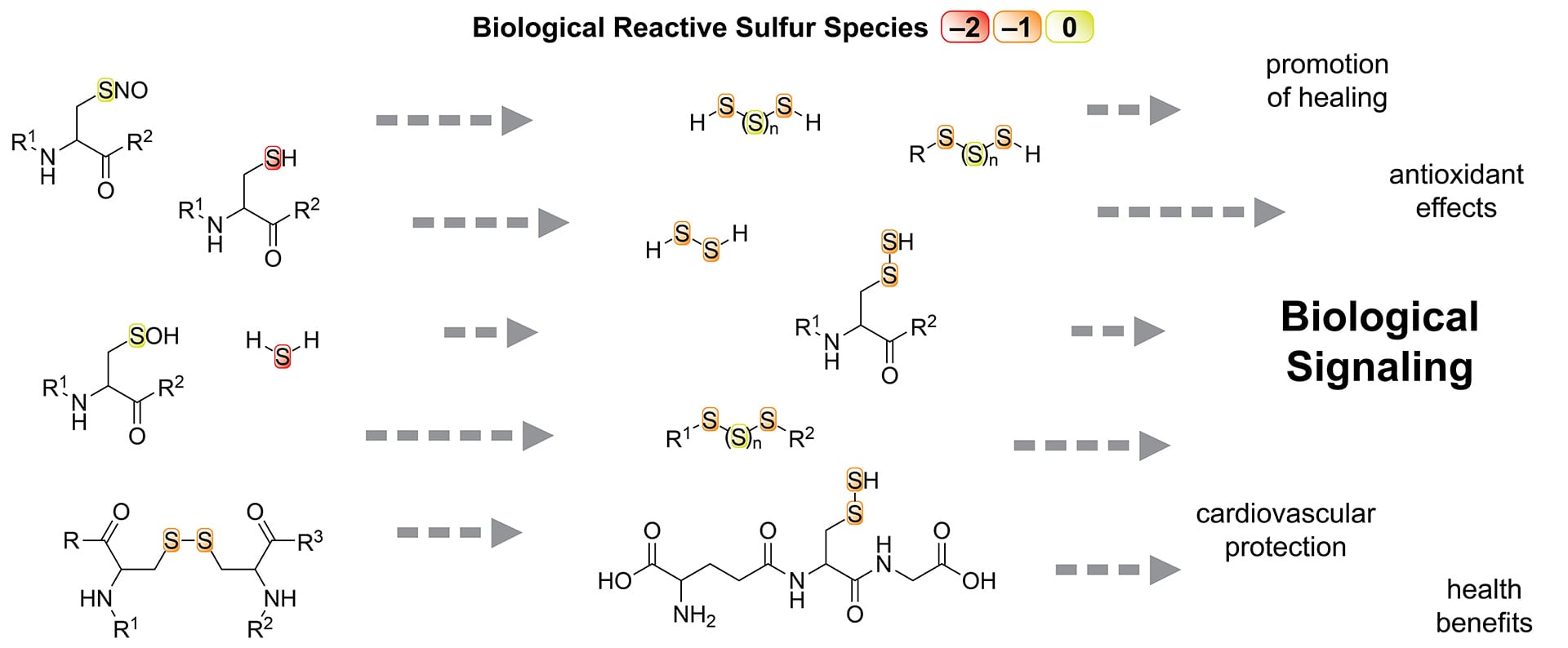
- Lau, N.; Pluth, M.D. “Reactive Sulfur Species (RSS): Persulfides, Polysulfides, Potential, and Problems” Curr. Opin. Chem. Biol. 2019, 48, 1-8. [10.1016/j.cbpa.2018.08.012]
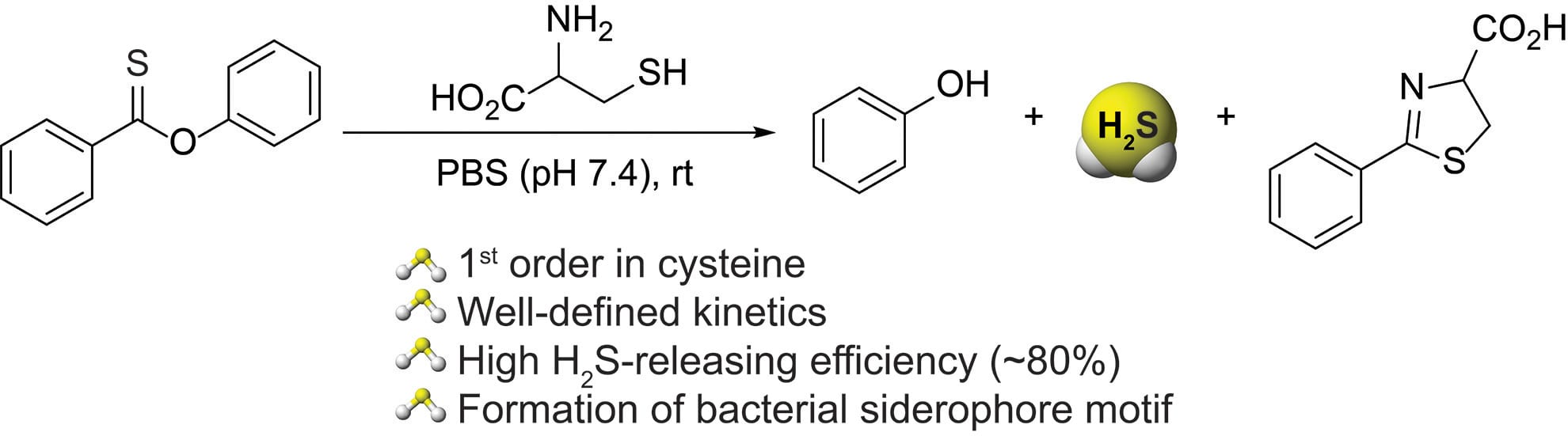
- Cerda, M.M.; Zhao, Y.; Pluth, M.D. “Thionoesters: A Native Chemical Ligation-Inspired Approach to Cysteine-Triggered H2S Donors” J. Am. Chem. Soc. 2018, 140(39), 12574-2579. [10.1021/jacs.8b07268]
– Highlighted by [Nature Chem. Biol.]
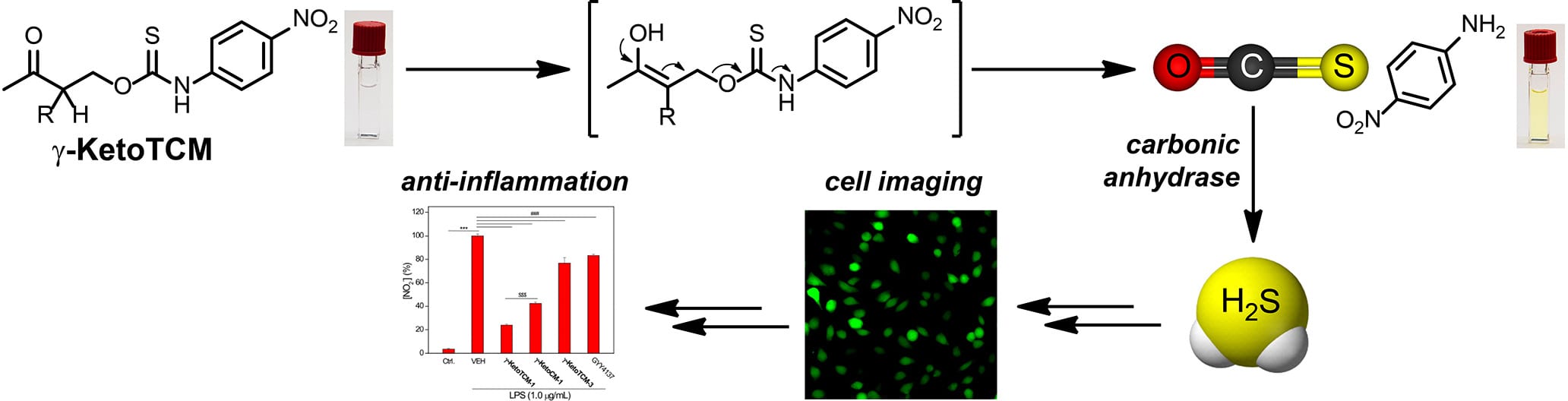
- Zhao, Y.; Steiger, A.K.; Pluth, M.D. “Colorimetric Carbonyl Sulfide (COS)/Hydrogen Sulfide (H2S) Donation from γ-Ketothiocarbamate Donor Motifs” Angew. Chem. Int. Ed. 2018, 57(40), 13101-13105. [10.1002/anie.201806854]
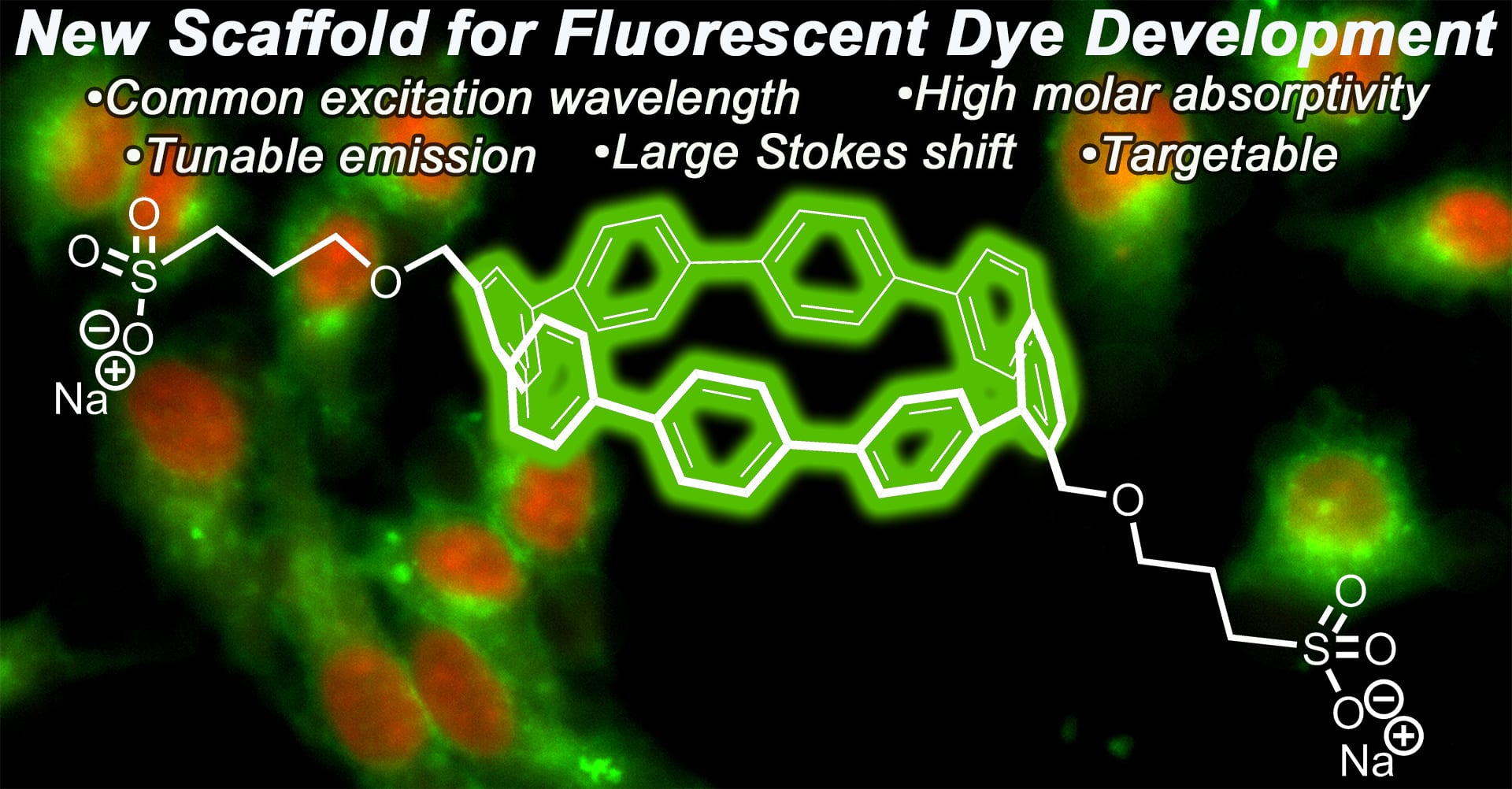
- White, B.M.; Zhao, Y.; Kawashima, T.E.; Branchaud, B.P.; Pluth, M.D.; Jasti, R. “Expanding the Chemical Space of Biocompatible Fluorophores: Nanohoops in Cells.” ACS Cent. Sci. 2018, 4(9), 1173-1178. [10.1021/acscentsci.8b00346]
– Highlighted by [EurekAlert!], [ScienceDaily], [Phys.org], [R&D]
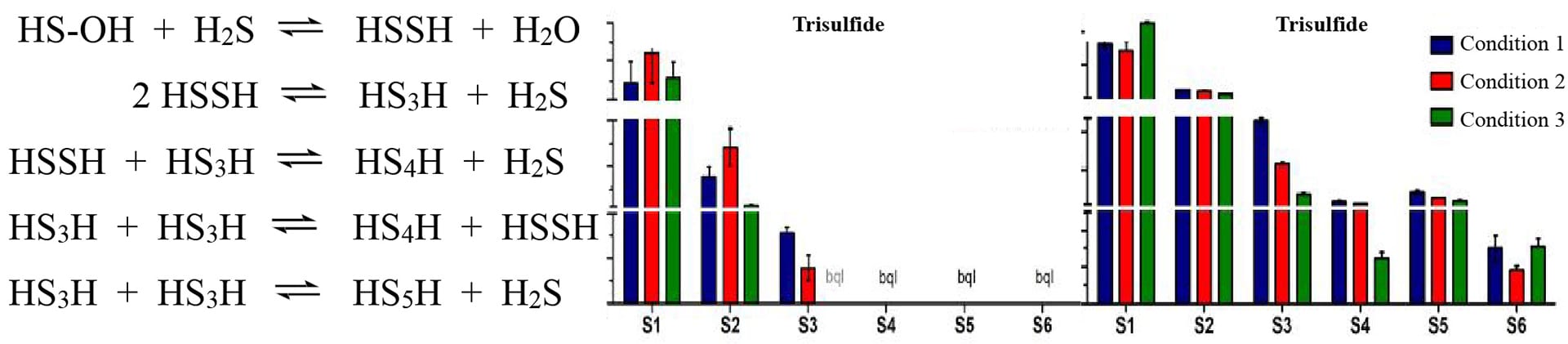
- Bogdándi, V.; Ida, T.; Sutton, T.R.; Bianco, C.; Ditrói, T.; Koster, G.; Henthorn, H.A.; Minnion, M.; Toscano, J.P.; van der Vliet, A.; Pluth, M.D.; Feelisch, M.; Fukuto, J.M.; Akaike, T.; Nagy, P. “Speciation of Reactive Sulfur Species and their Reactions with Alkylating Agents: Do we Have any Clue About what is Present Inside the Cell?” Br. J. Pharmacol. 2019, 176(4), 646-670. [10.1111/bph.14394]
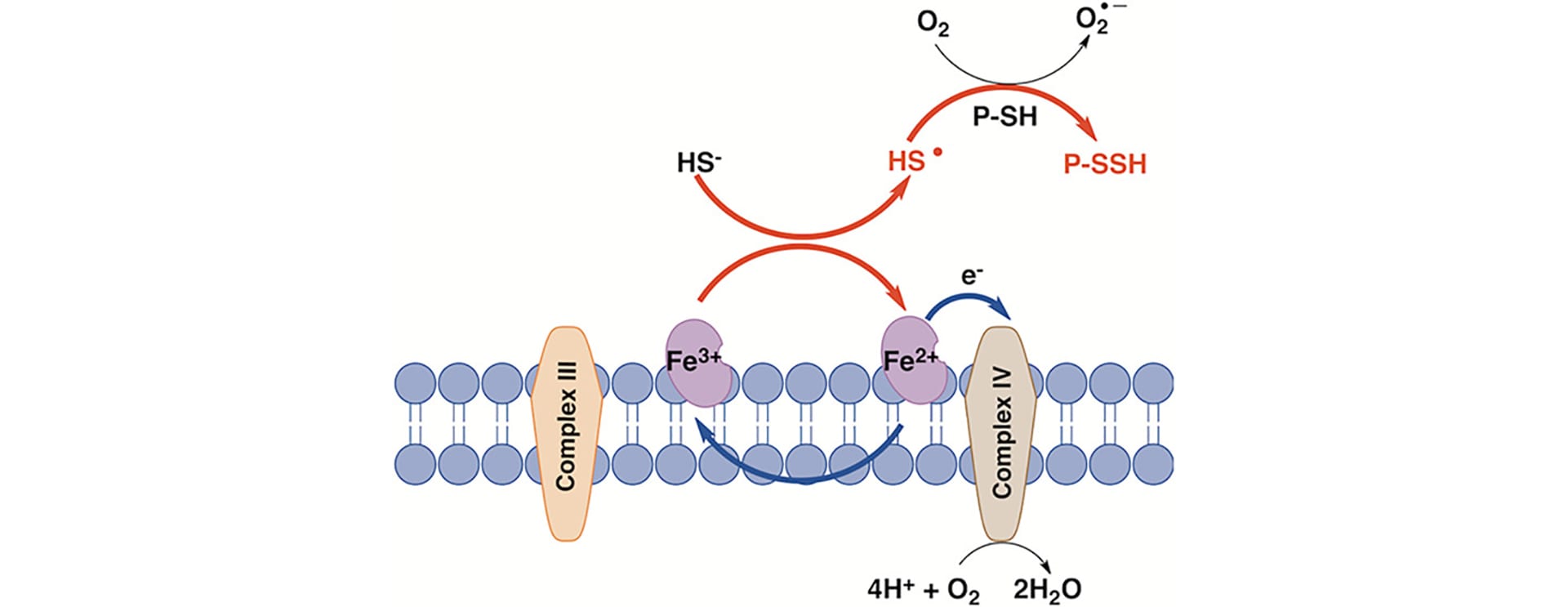
- Vitvitsky , V.; Miljkovic, J.L.; Bostelaar, T.; Adhikari, B.; Yadav, P.K.; Steiger, A.K.; Torregrossa, R.; Pluth, M.D.; Whiteman, M.; Banerjee, R.; Filipovic, M.R. “Cytochrome c Reduction by H2S Potentiates Sulfide Signaling.” ACS Chem. Biol. 2018, 13(8), 2300-2307. [10.1021/acschembio.8b00463]
- Zhao, Y.; Steiger, A.K., Pluth, M.D. “Cysteine-Activated Hydrogen Sulfide (H2S) Delivery through Caged Carbonyl Sulfide (COS) Donor Motifs” Chem. Commun. 2018, 54, 4951-4954. [10.1039/C8CC02428F]
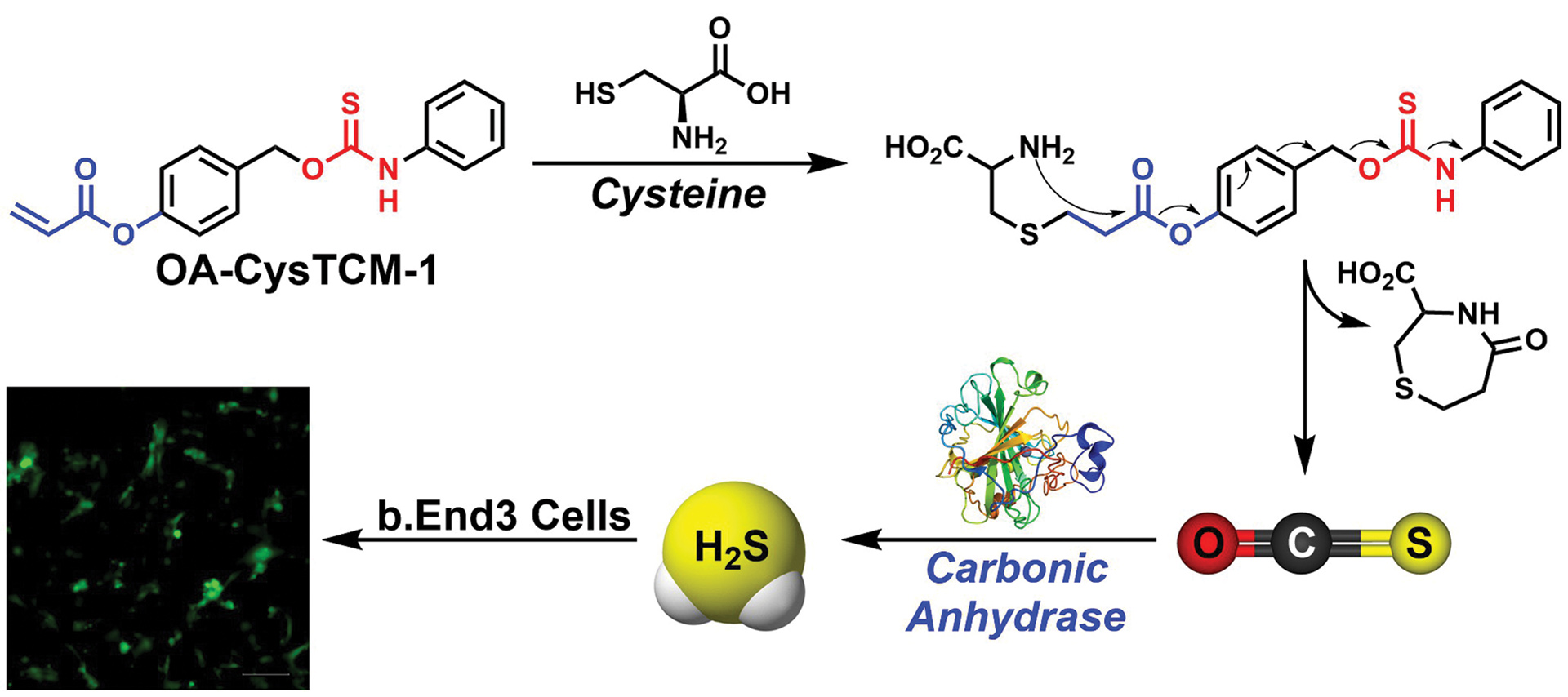
- Cerda, M.M.; Pluth, M.D. “S Marks the Spot: Linking the Antioxidant Activity of N-Acetyl Cysteine to H2S and Sulfane Sulfur Species” Cell Chem. Biol. 2018, 25(4), 353-355. [10.1016/j.chembiol.2018.04.001] (Preview of T. Dick paper on NAC Activity)
- Lau, N.; Zakharov, L.N.; Pluth, M.D. “Modular Tripodal Receptors for the Hydrosulfide (HS–) Anion” Chem. Commun. 2018, 54, 2337-2340. [10.1039/C7CC09405A]
– Cover article

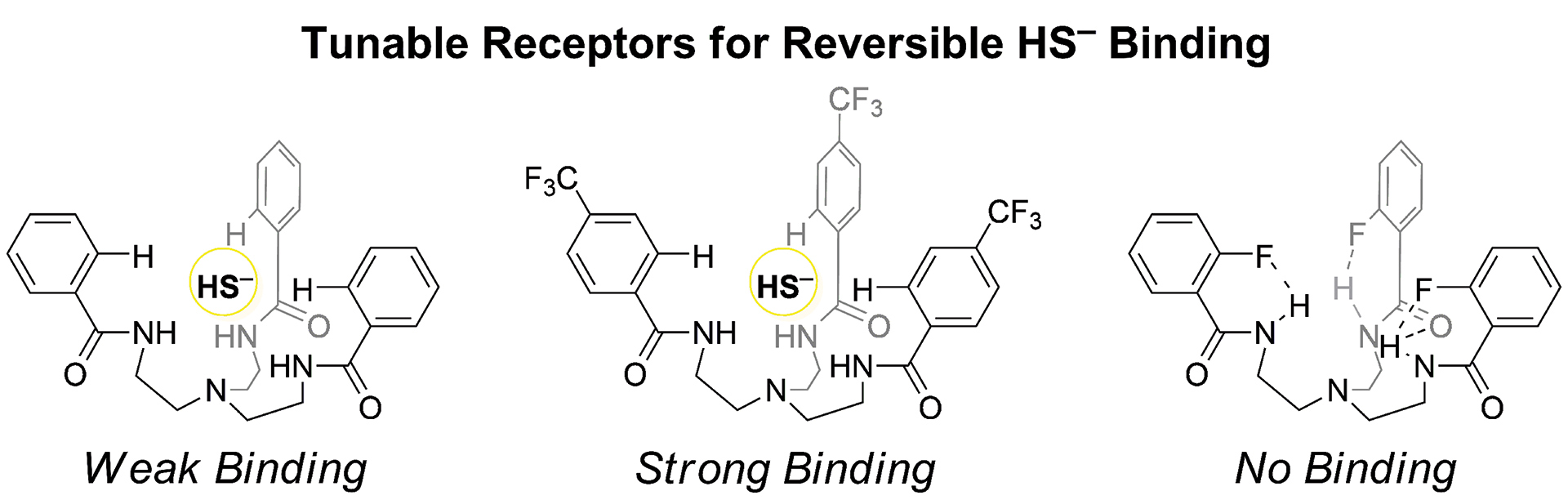
- Steiger, A.K.; Zhao, Y.; Choi, W.J.; Tillotson, M.R.; Crammond, A.; Pluth, M.D. “Investigations into the Carbonic Anhydrase Inhibition of COS-Releasing Donor Core Motifs” Biochem. Pharmacol. 2017, 149, 124-130. [10.1016/j.bcp.2017.11.004]
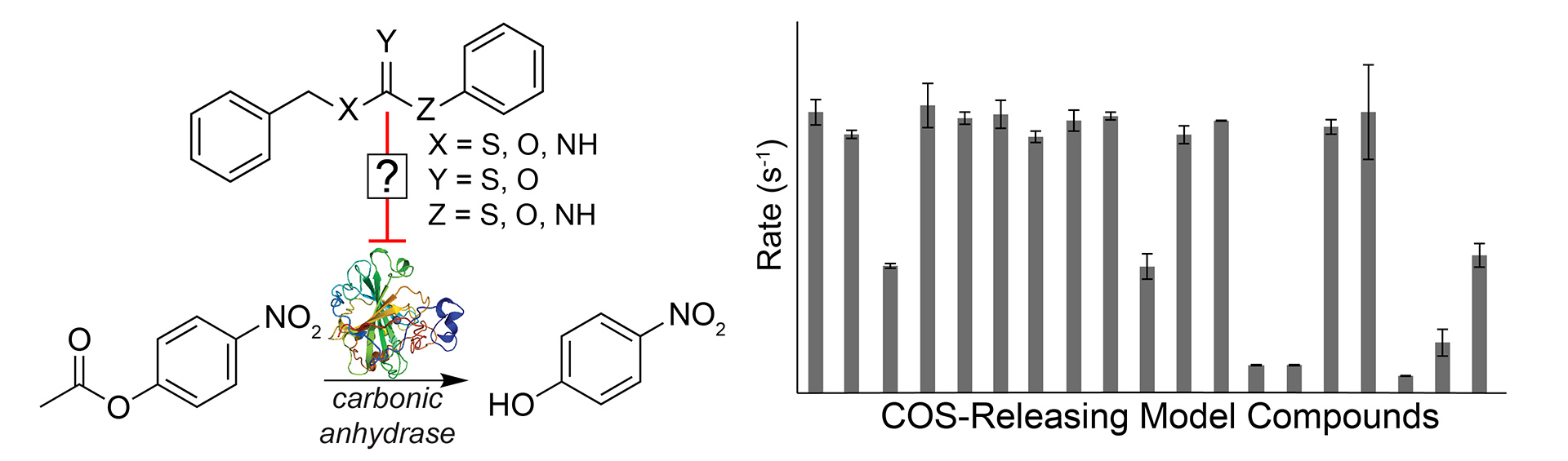
- Sgarlata, C.; Mugridge, J.S.; Pluth, M.D.; Zito, V.; Arena, G.; Raymond, K.N. “Different and Often Opposing Forces Drive the Encapsulation and Multiple Exterior Binding of Charged Guests to a M4L6 Supramolecular Vessel in Water” Chem. Eur. J. 2017, 23, 16813-16818. [10.1002/chem.201703202]
– “Hot Paper” highlighted by Angew. Chem. Int. Ed.
- Zhao, Y.; Henthorn, H.A.; Pluth, M.D. “Kinetic Insights into Hydrogen Sulfide Delivery from Caged Carbonyl Sulfide Isomeric Donor Platforms” J. Am. Chem. Soc. 2017, 139(45), 16365-16376. [10.1021/jacs.7b09527]
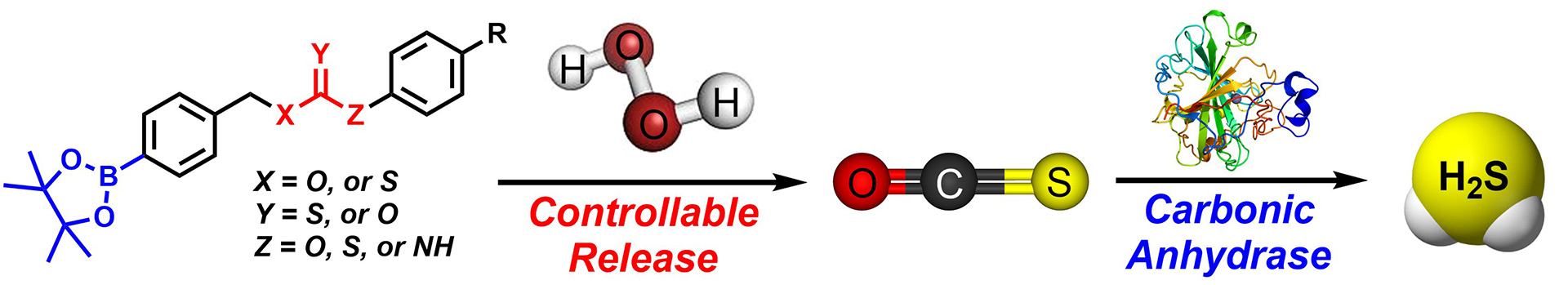
–
 Recommended on Faculty1000 Prime
Recommended on Faculty1000 Prime
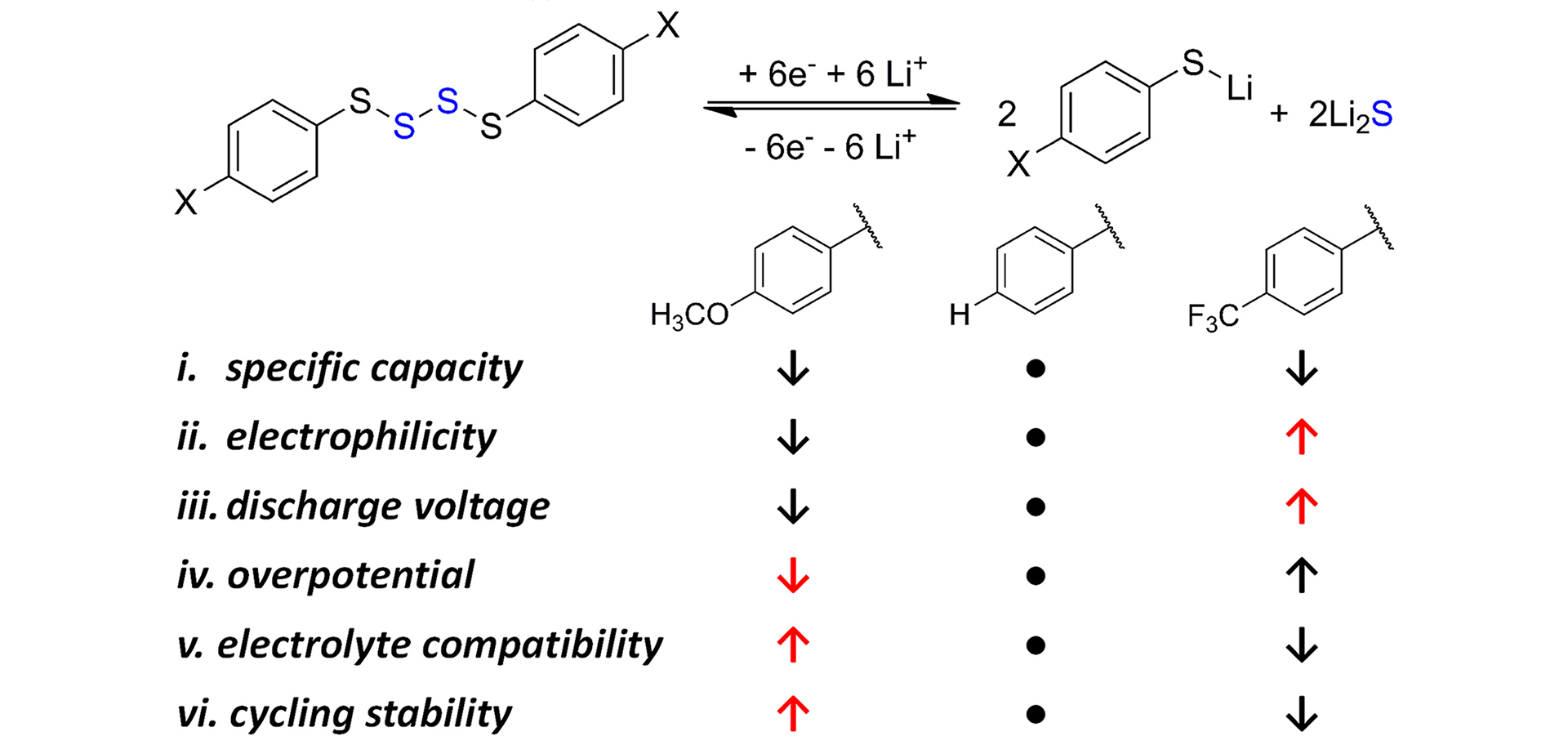
- Guo, W.; Wawrzyniakowski, Z.D.; Cerda, M.M.; Bhargav, A.; Pluth, M.D.; Ma, Y.; Fu, Y. “Bis(aryl) Tetrasulfides as Cathode Materials for Rechargeable Lithium Batteries” Chem. Eur. J. 2017, 23, 16941-16947. [10.1002/chem.201703895]
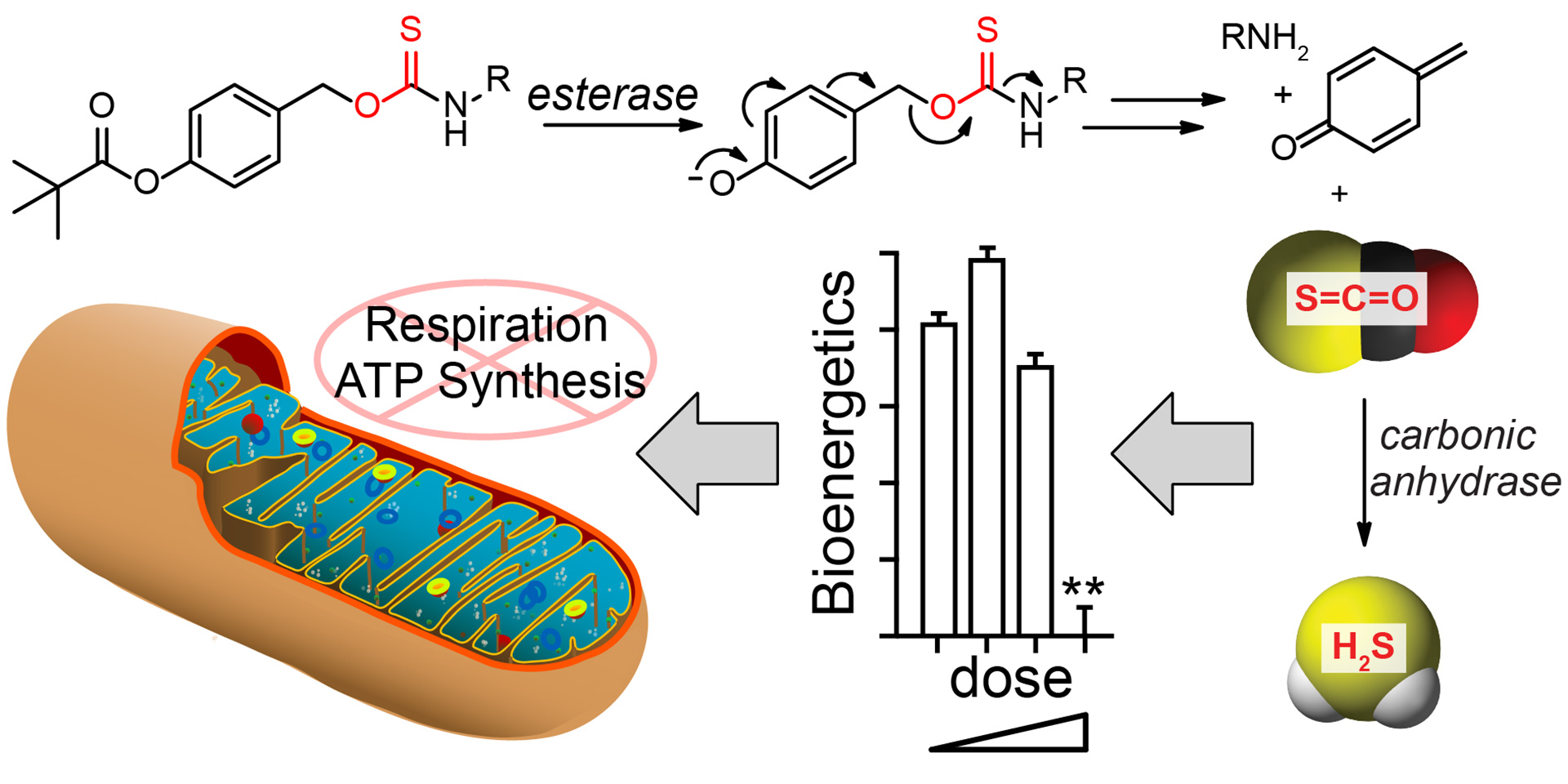
- Steiger, A.K.; Marcatti, M.; Szabo, C.; Szczesny, B.; Pluth, M.D. “Inhibition of Mitochondrial Bioenergetics by Esterase-Triggered COS/H2S Donors” ACS Chem. Biol. 2017, 12(8), 2117-2123. [10.1021/acschembio.7b00279]
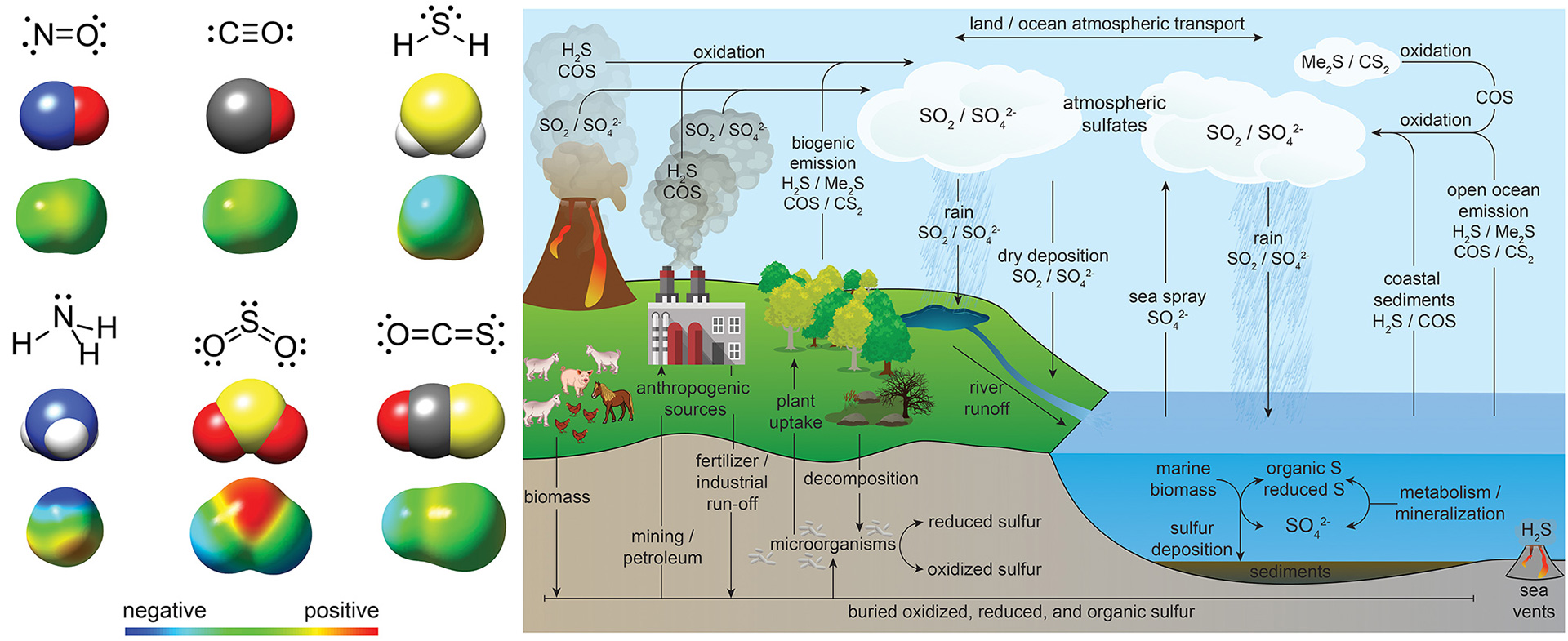
- Steiger, A.K.; Zhao, Y.; Pluth, M.D. “Emerging Roles of Carbonyl Sulfide (COS) in Chemical Biology: Sulfide Transporter or Gasotransmitter?” Antioxid. Redox Signal. 2018, 28(16), 1516-1532. [10.1089/ars.2017.7119]
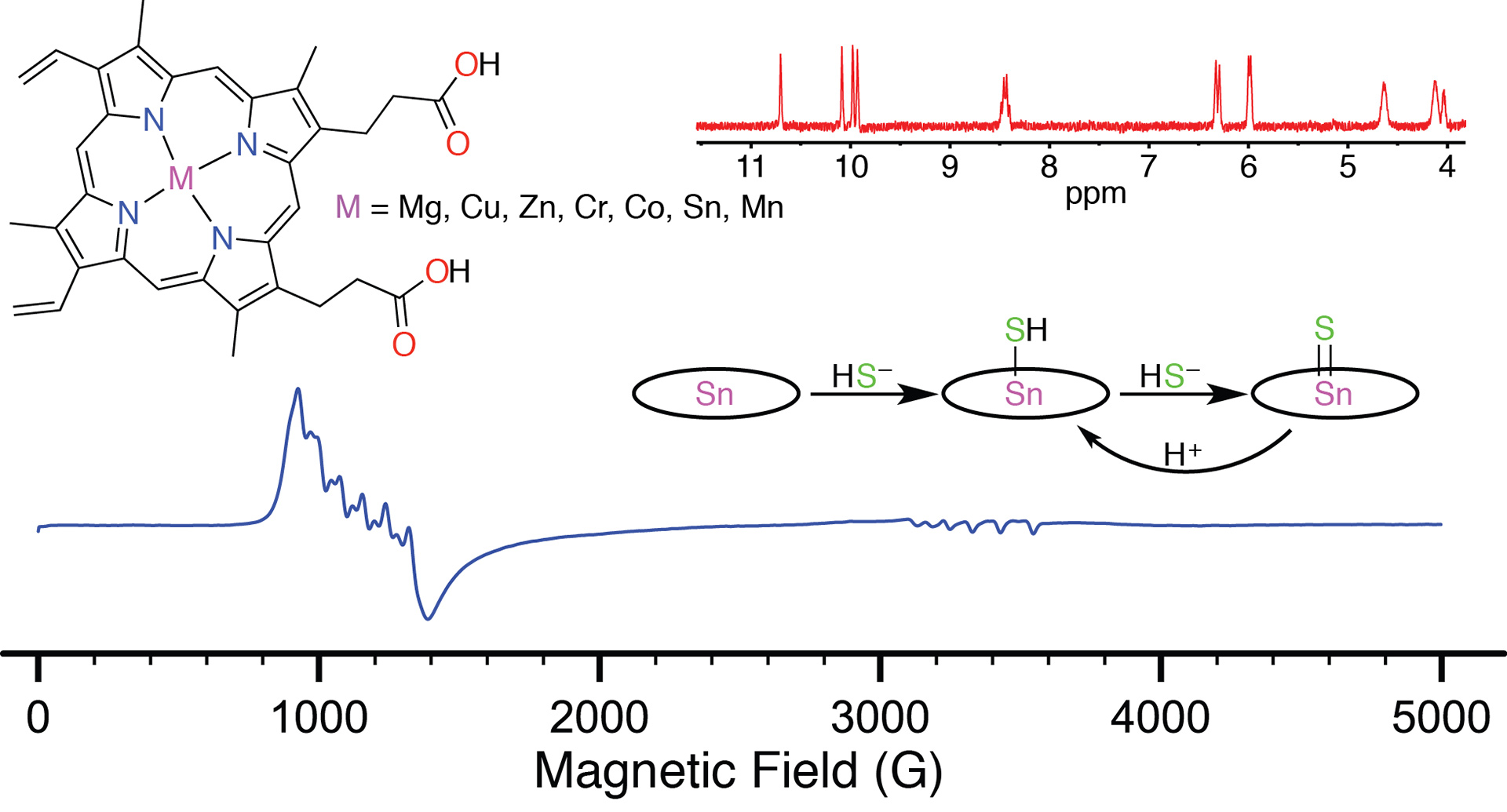
- Hartle, M.D.; Tillotson, M.R.; Prell, J.S.; Pluth, M.D. “Spectroscopic Investigation of the Reaction of Metallo-protoporphyrins with Hydrogen Sulfide.” J. Inorg. Biochem. 2017, 173, 152-157. [10.1016/j.jinorgbio.2017.04.021]
- Cerda, M.M.; Hammers, M.D.; Earp, M.S.; Zakharov, L.N.; Pluth, M.D. “Applications of Synthetic Organic Tetrasulfides as H2S Donors.” Org. Lett. 2017, 19(9), 2314-2317. [10.1021/acs.orglett.7b00858]
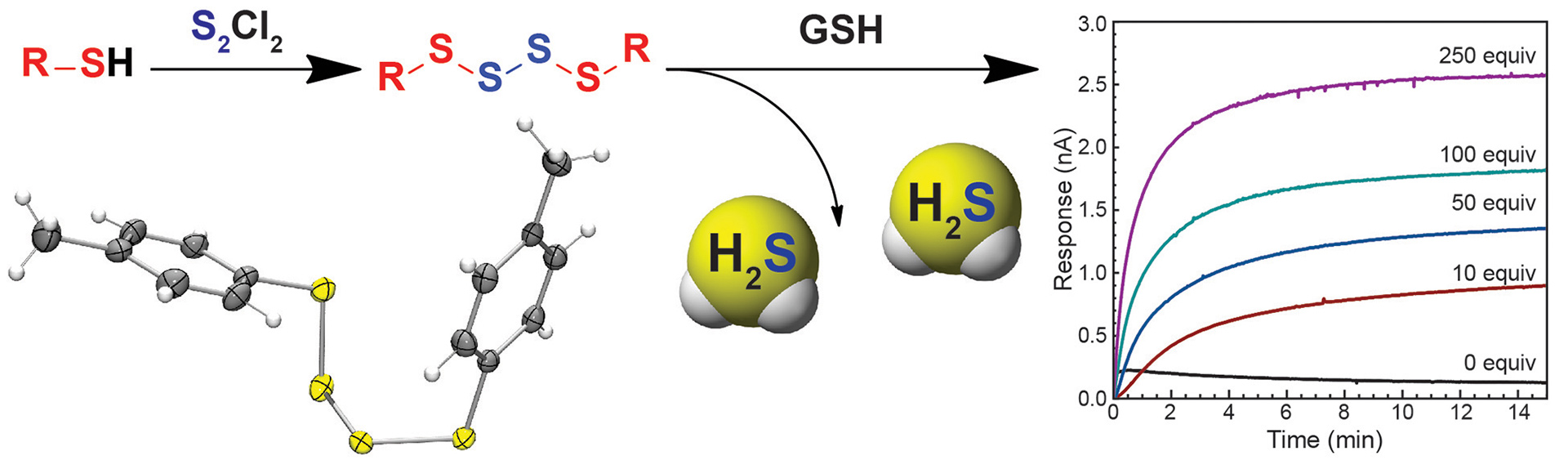
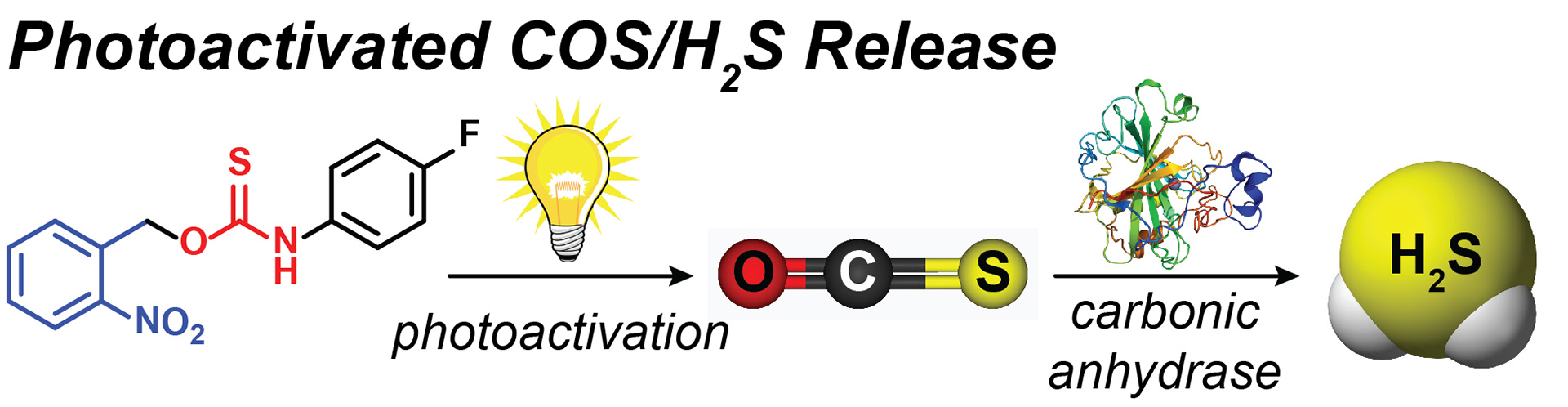
- Zhao, Y.; Bolton, S.G.; Pluth, M.D. “Light-Activated COS/H2S Donation from Photocaged Thiocarbamates.” Org. Lett. 2017, 19(9), 2278-2281. [acs.orglett.7b00808]
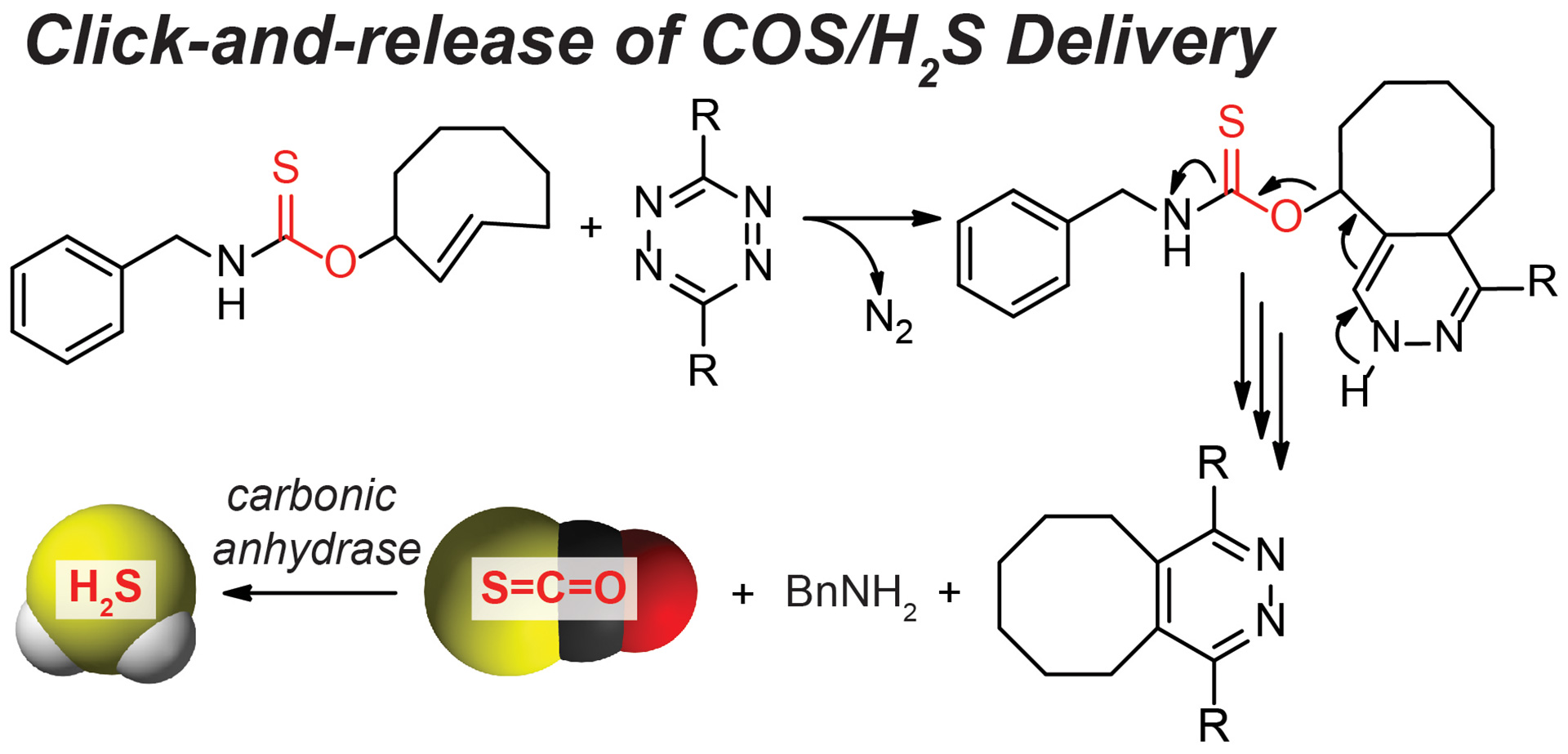
- Steiger, A.K.; Yang, Y.; Royzen, M.; Pluth, M.D. “Bio-orthogonal “Click-and-Release” Donation of Caged Carbonyl Sulfide (COS) and Hydrogen Sulfide (H2S).” Chem. Commun. 2017, 53, 1378-1380. [10.1039/C6CC09547J]
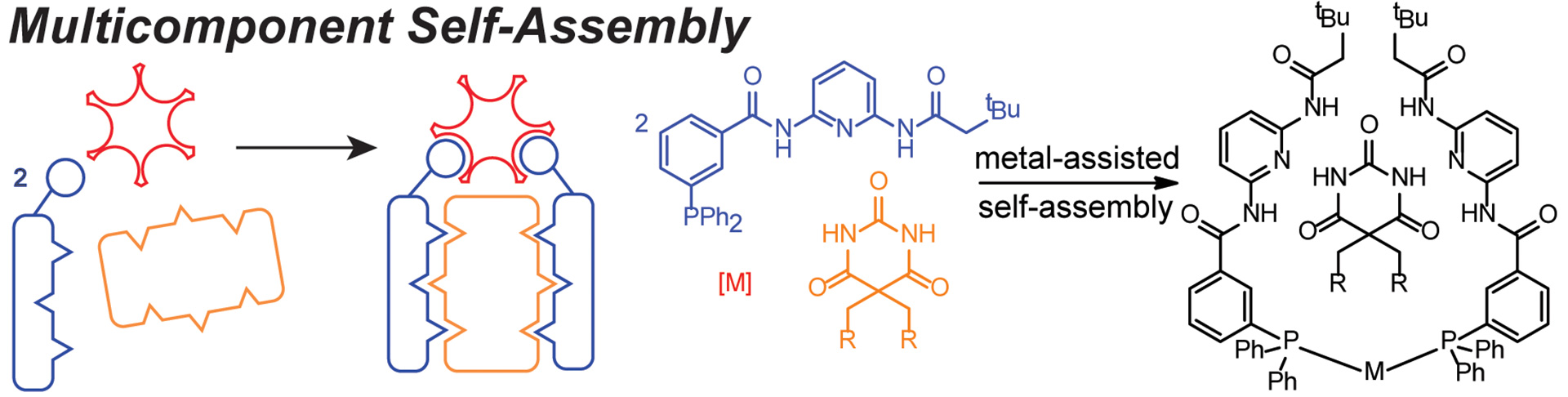
- Seidenkranz, D.T.; McGrath, J.M.; Zakharov, L.N.; Pluth, M.D. “Supramolecular Bidentate Phosphine Ligand Scaffolds from Deconstructed Hamilton Receptors.” Chem. Commun. 2017, 53, 561-564. [10.1039/C6CC09198A]
- Bailey, T.S.; Henthorn, H.A.; Pluth, M.D. “The Intersection of NO and H2S: Persulfides Generate NO from Nitrite through Polysulfide Formation.” Inorg. Chem. 2016, 55(24), 12618-12625. [10.1021/acs.inorgchem.6b01660]
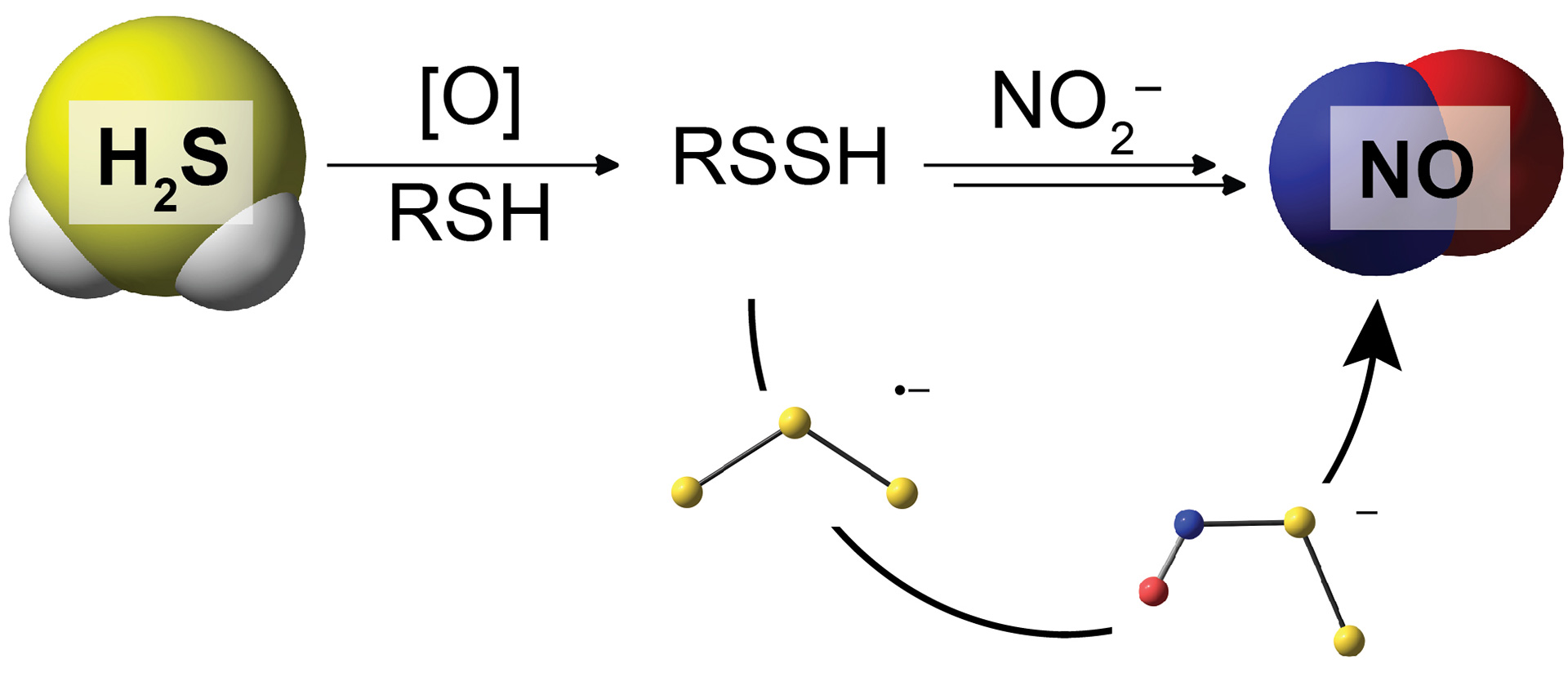
- Zhao, Y.; Pluth, M.D. “Hydrogen Sulfide Donors Activated by Reactive Oxygen Species.” Angew. Chem. Int. Ed. 2016, 55(47) 14638-14642. [10.1002/anie.201608052]
– Highlighted by [AroundTheO], [Phys.org], [ScienceBlog], [EurekaAlert!], [ChemEurope] [Parkinson’s News Today]
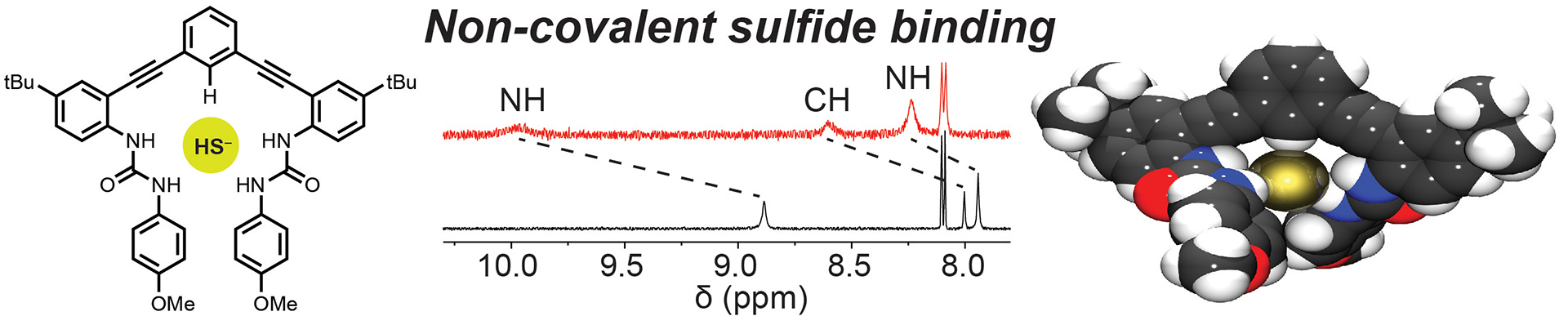
- Hartle, M.D.; Hansen, R.J.; Tresca, B.W.; Prakel, S.S.; Zakharov, L.N.; Haley, M.M.; Pluth, M.D.; Johnson, D.W. “A Synthetic Supramolecular Receptor for the Hydrosulfide Anion.” Angew. Chem. Int. Ed. 2016, 55(38), 11480-11484. [10.1002/anie.201605757]
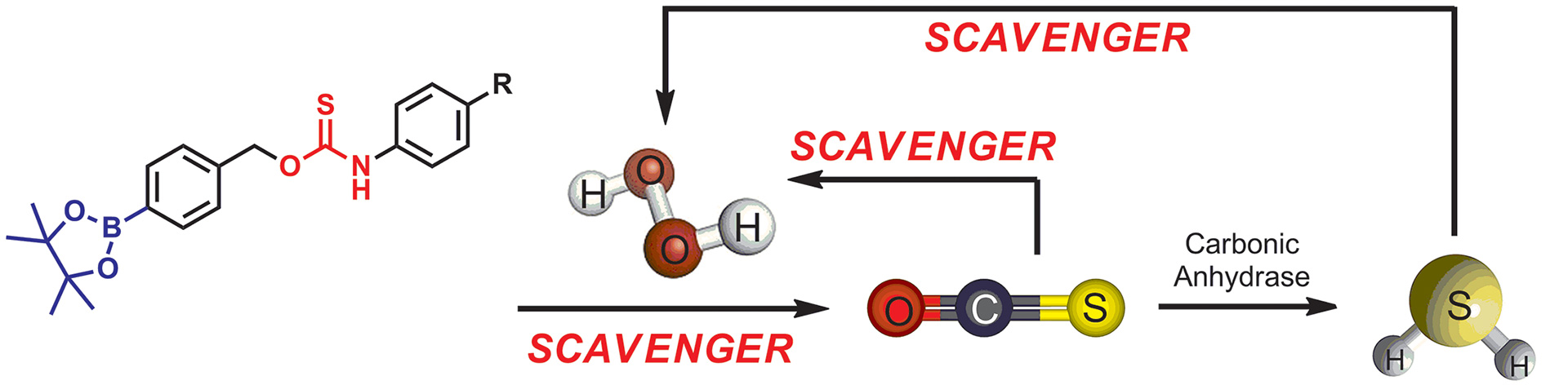
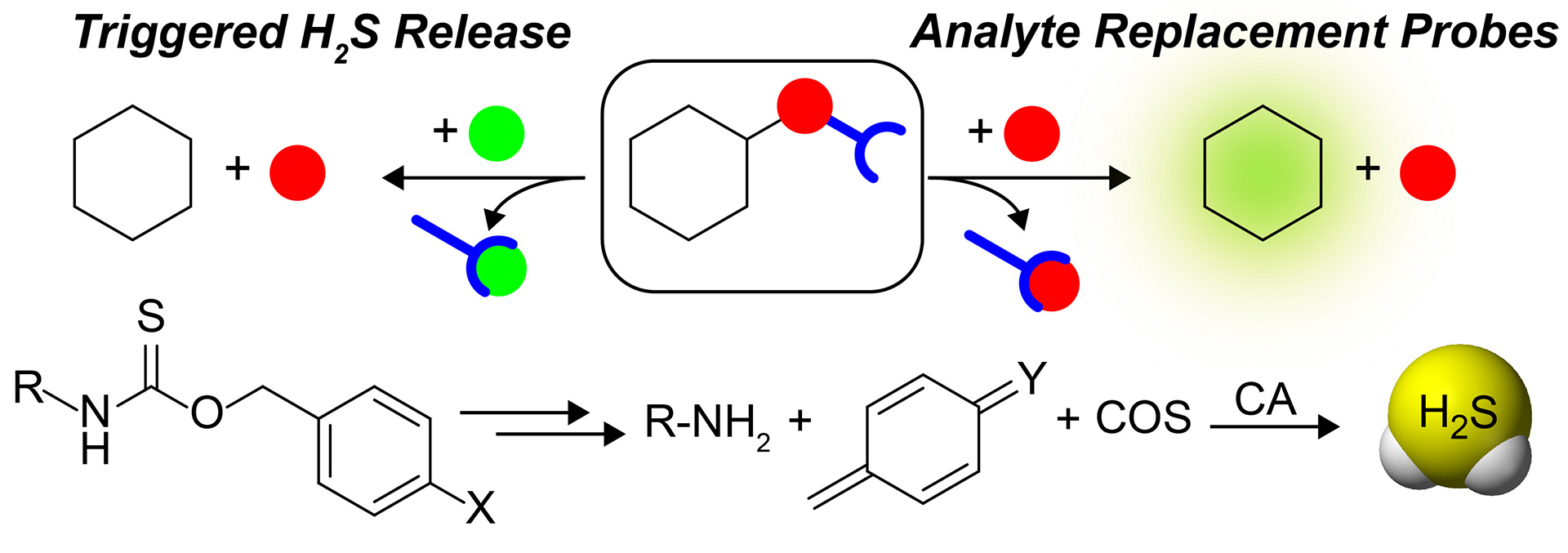
- Steiger, A.K.; Pardue, S.; Kevil, C.G.; Pluth, M.D. “Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes.” J. Am. Chem. Soc. 2016, 138, 7256-7259. [10.1021/jacs.6b03780]
– Highlighted by [AroundTheO], [Phys.org], [ScienceBlog], [EurekaAlert!], [ChemEurope], [Parkinson’s News Today]
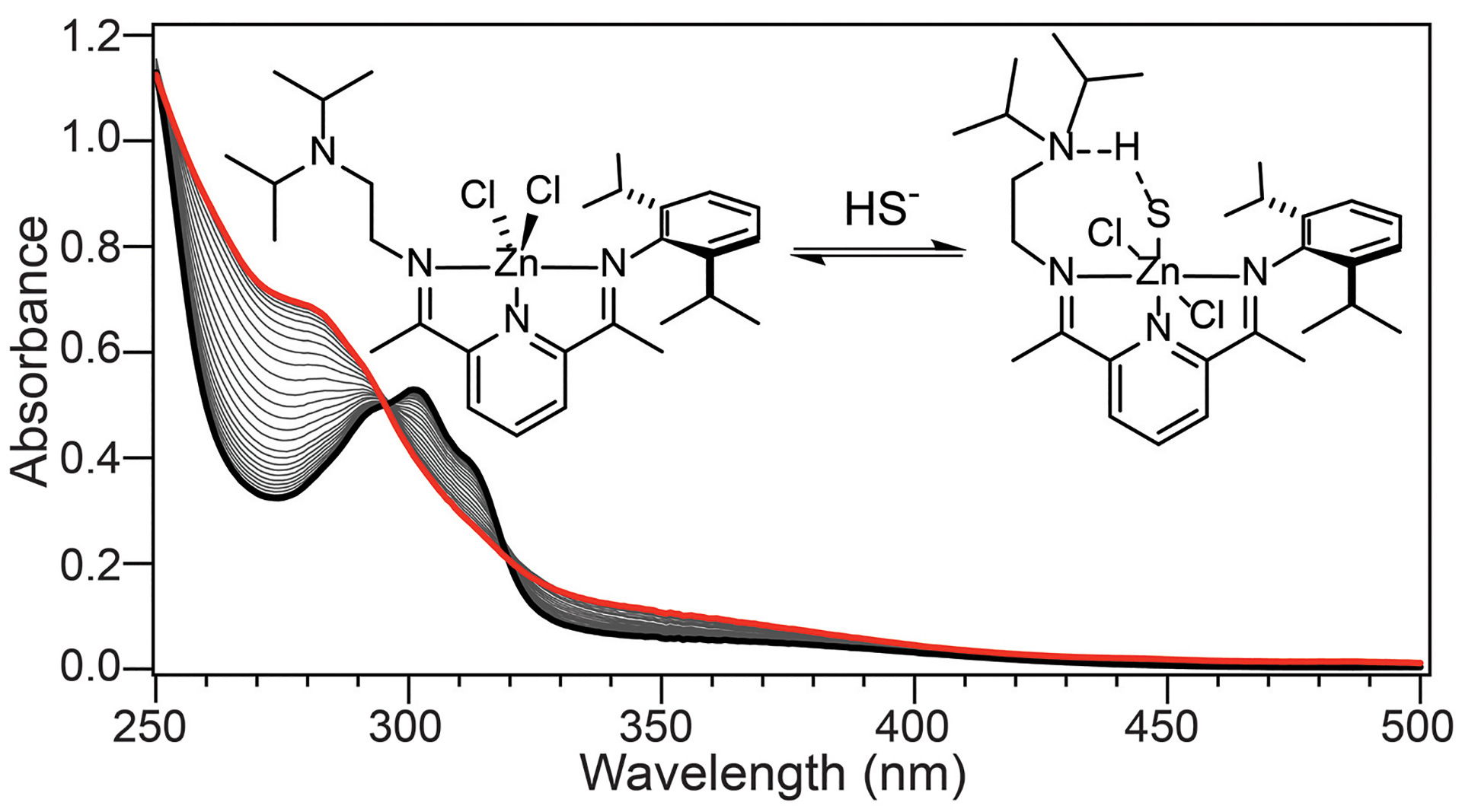
- Hartle, M.D.; Delgado, M.; Gilbertson, J.D.; Pluth, M.D. “Stabilization of a Zn(II) Hydrosulfido Complex Utilizing a Hydrogen-Bond Accepting Ligand.” Chem. Commun. 2016, 52, 7680-7682. [10.1039/C6CC01373B]
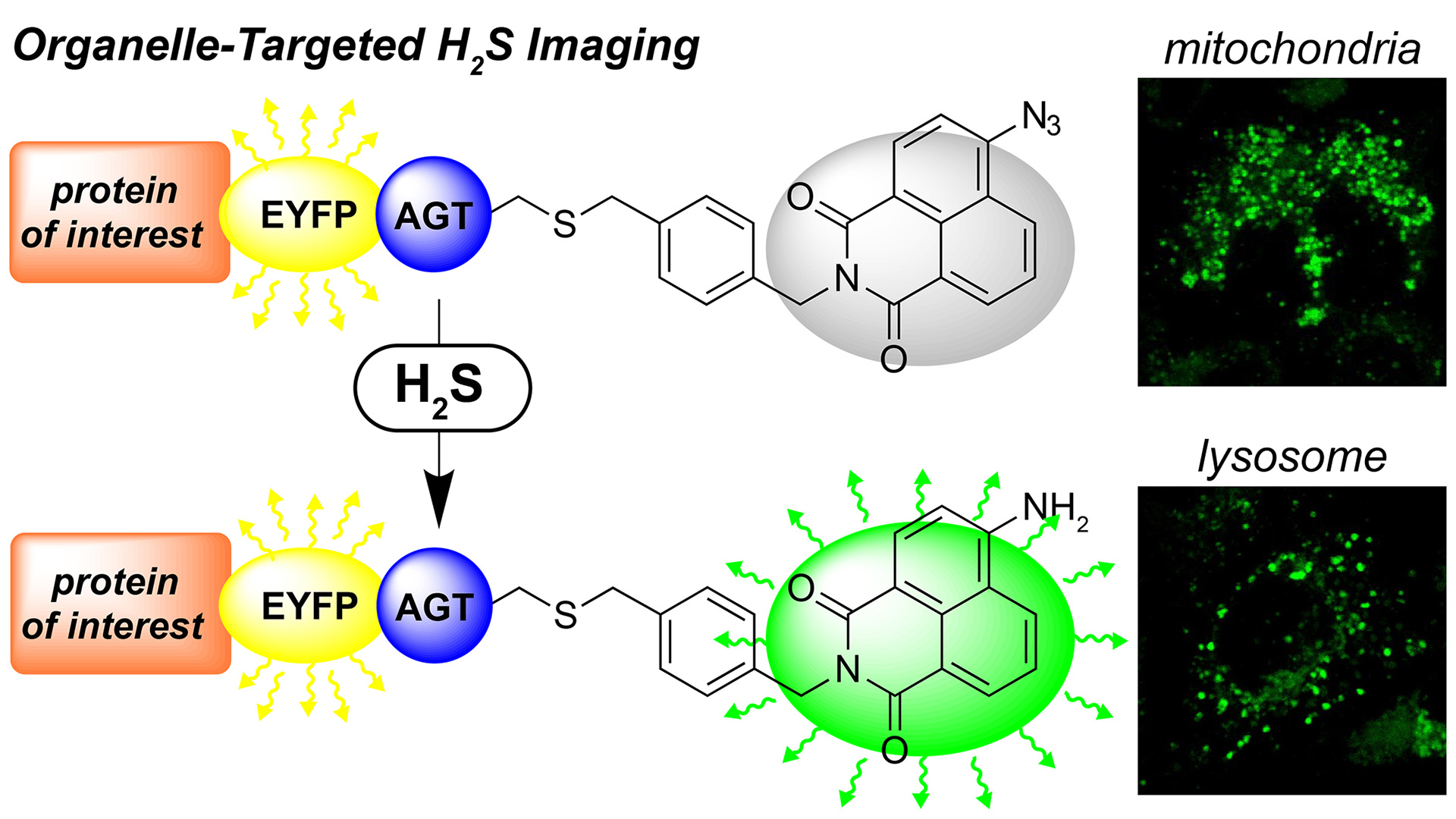
- Montoya, L.A.; Pluth, M.D. “Organelle-Targeted H2S Probes Enable Visualization of the Subcellular Distribution of H2S Donors.” Anal. Chem. 2016, 88(11), 5769-5774. [10.1021/acs.analchem.6b00087]
- Hartle, M.D.; Pluth, M.D. “A Practical Guide to Working with H2S at the Interface of Chemistry and Biology.” Chem. Soc. Rev. 2016, 45, 6108-6117. [10.1039/C6CS00212A]
– Cover article

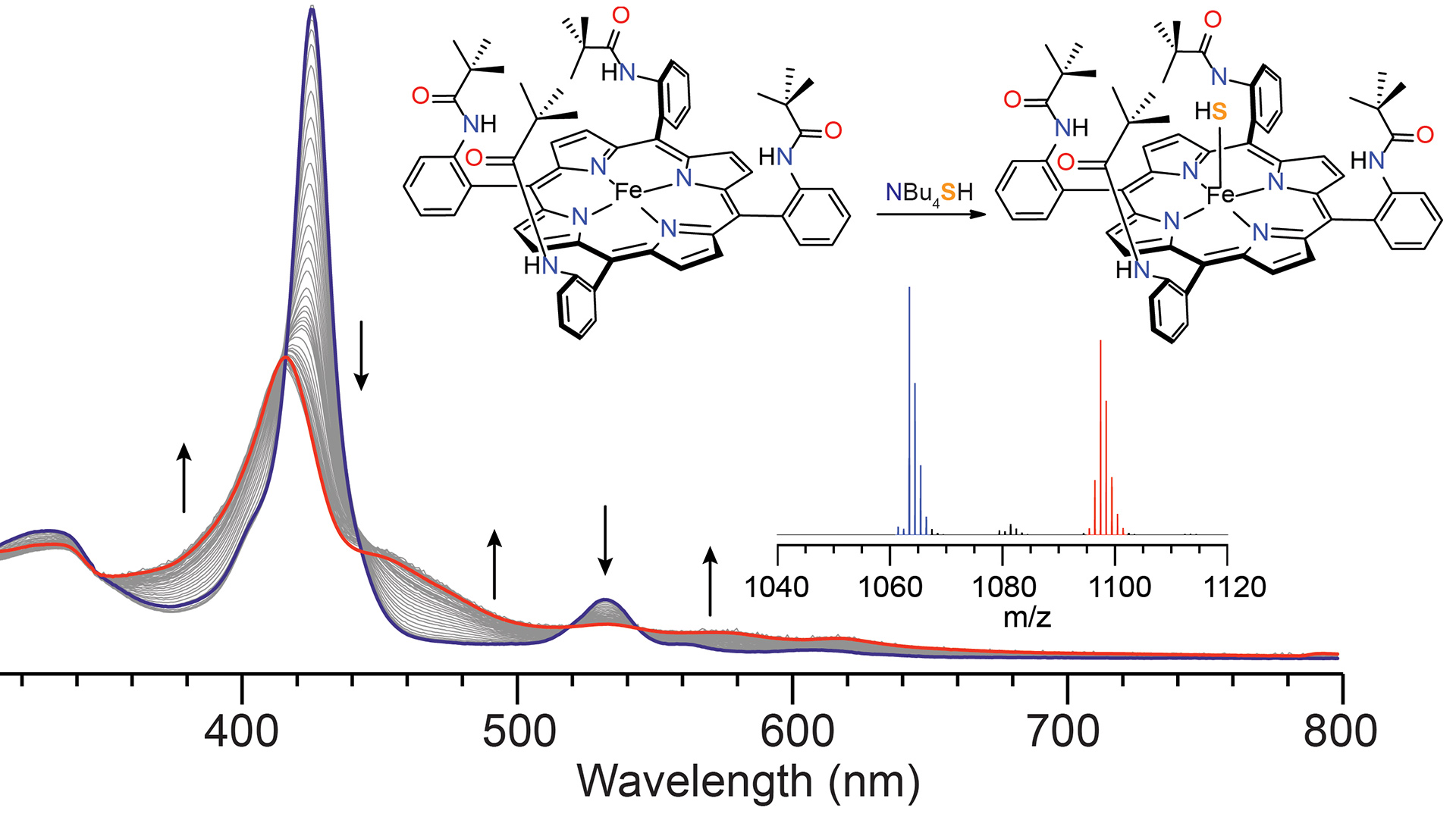
- Hartle, M.D.; Prell, J.S.; Pluth, M.D. “Spectroscopic Investigations into the Binding of Hydrogen Sulfide to Synthetic Picket-Fence Porphyrins.” Dalton Trans. 2016, 45, 4843-4853. [10.1039/C5DT04563K]
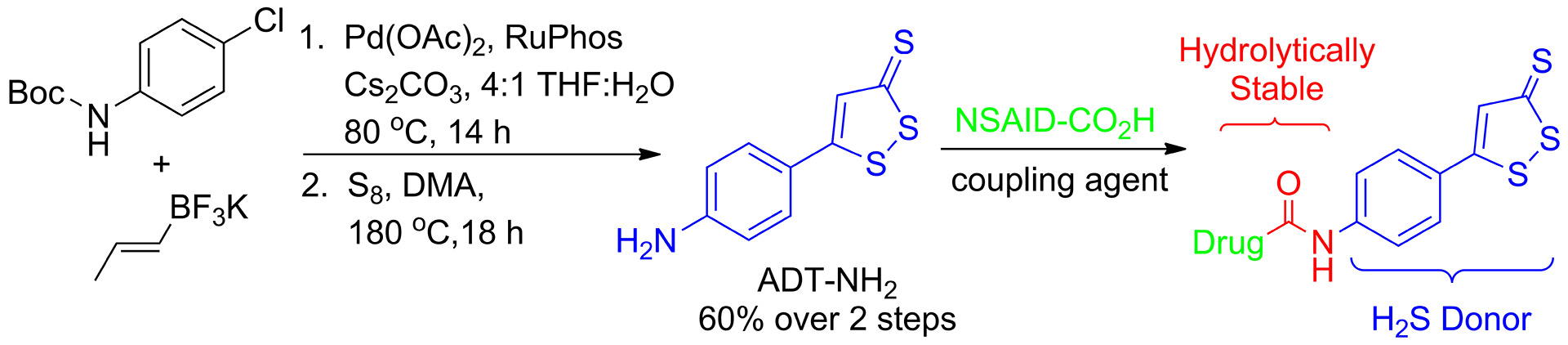
- Hammers, M.D.; Singh, L.; Montoya, L.A.; Moghaddam, A.D.; Pluth, M.D. “Synthesis of Amino-ADT Provides Access to Hydrolytically-Stable Amide-Coupled Hydrogen Sulfide-Releasing Drug Targets.” Synlett. 2016, 27(9), 1349-1353. [10.1055/s-0035-1560603]
- Yang, G.; Sener, A.; Ji, Y.; Pei, Y.; Pluth, M.D. “Gasotransmitters in Biology and Medicine: Molecular Mechanisms and Drug Targets.” Oxid. Med. Cell. Longev. 2016, 2016, 4627308. [10.1155/2016/4627308]
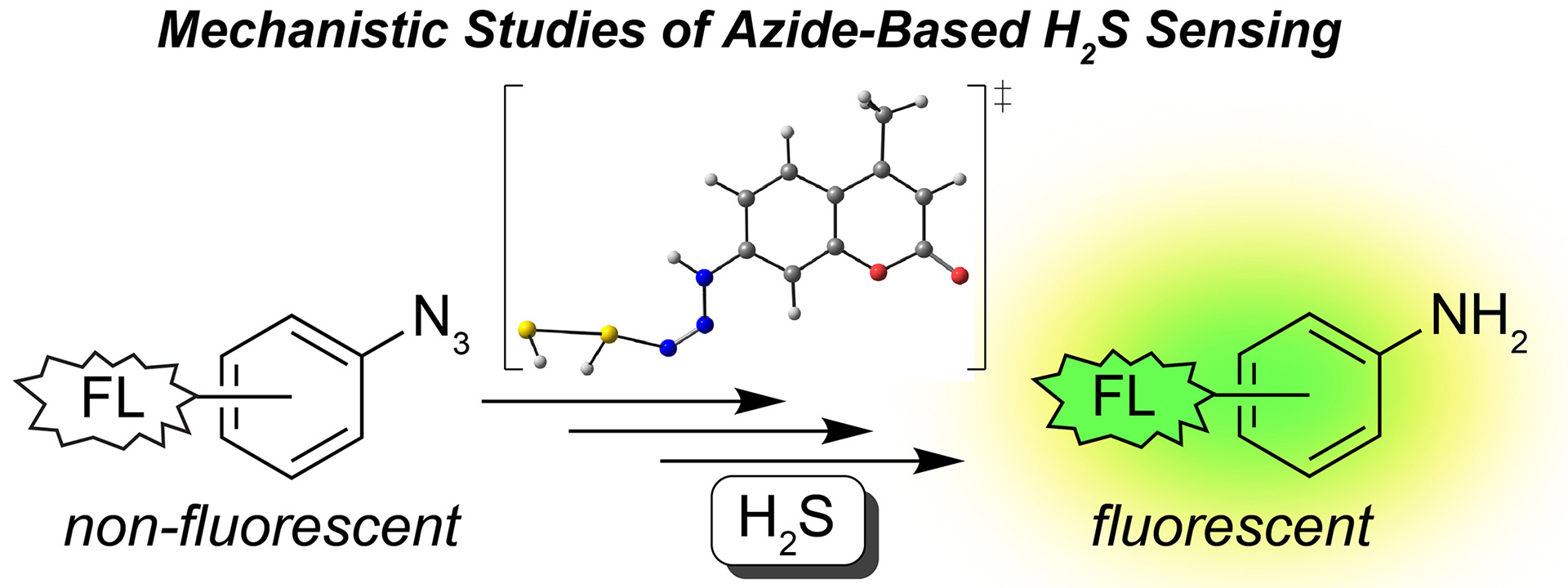
- Henthorn, H.A.; Pluth, M.D. “Mechanistic Insights into the H2S-Mediated Reduction of Aryl Azides Commonly used in H2S Detection.” J. Am. Chem. Soc. 2015, 137(48), 15330-15336. [10.1021/jacs.5b10675]
– JACS Spotlight Article
- Hartle, M.D.; Meininger, D.J.; Zakharov, L.N.; Tonzetich, Z.J.; Pluth, M.D. “NBu4SH Provides a Convenient Source of HS–Soluble in Organic Solution for H2S and Anion-Binding Research.” Dalton. Trans. 2015, 44, 19782-19785. [10.1039/C5DT03355A]
– Dalton Trans. Hot Article, 2016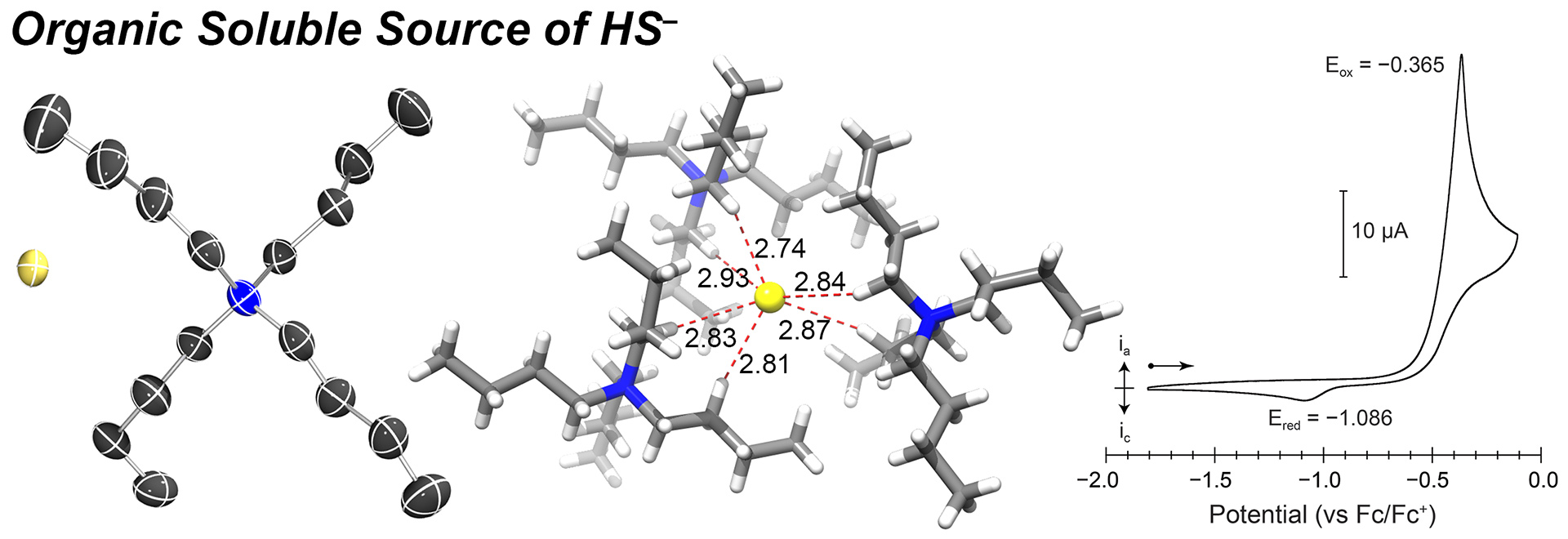
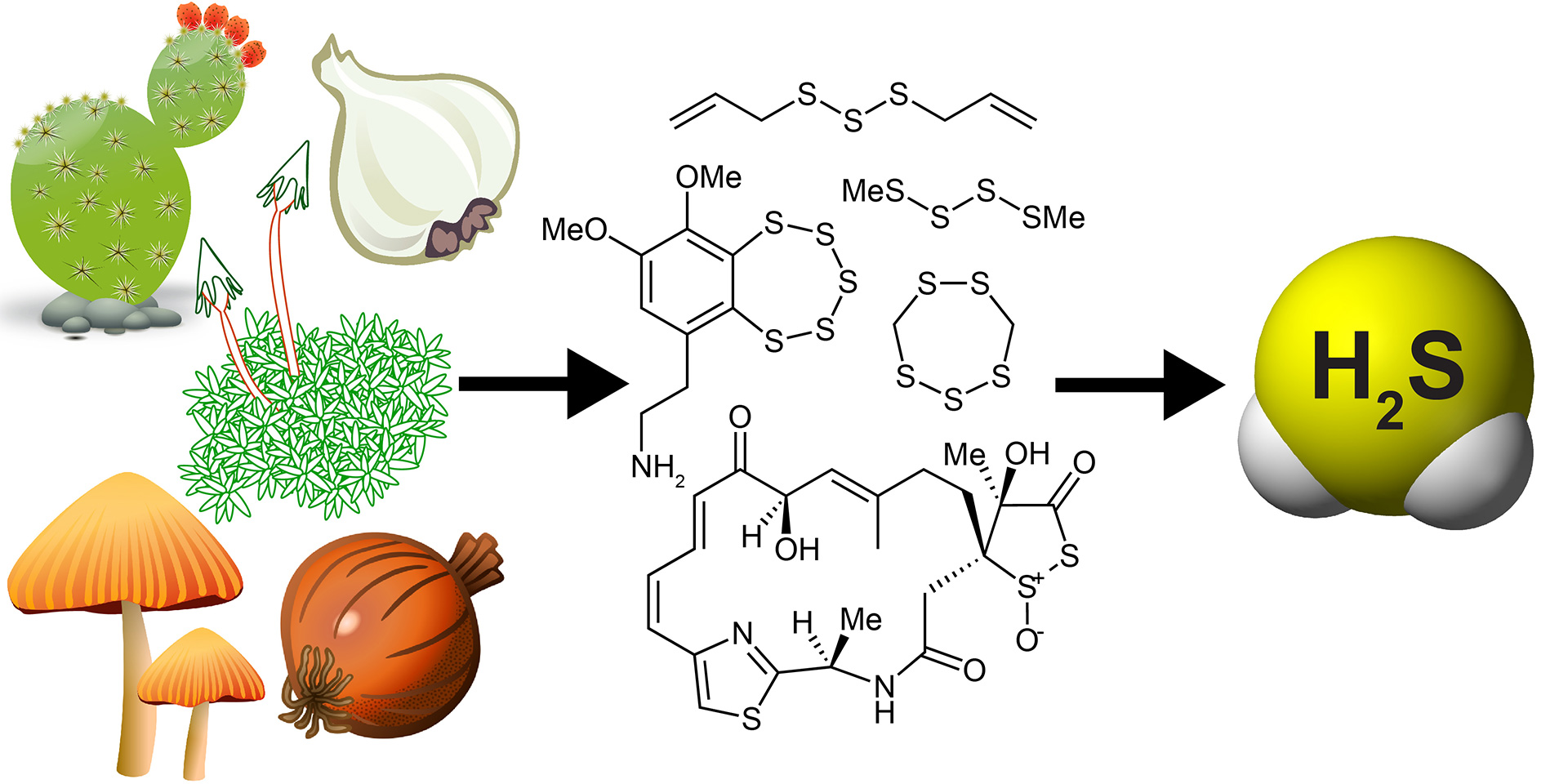
- Pluth, M.D.; Bailey, T.S.; Hammers, M.D.; Hartle, M.D.; Henthorn, H.A.; Steiger, A.K. “Natural Products Containing Hydrogen Sulfide Releasing Moieties.” Synlett 2015, 26(19), 2633-2643. [10.1055/s-0035-1560638]
– Second most downloaded Synlett paper, Dec. 2015.
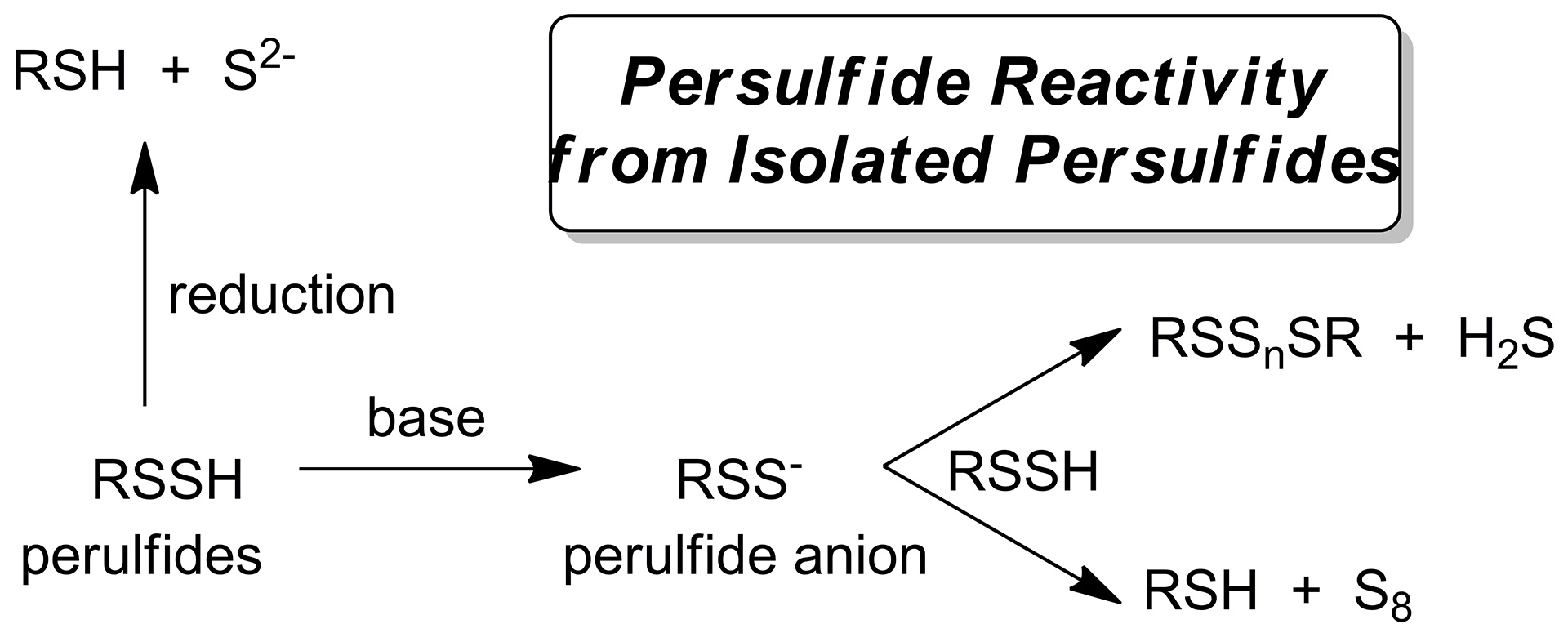
- Bailey, T.S.; Pluth, M.D. “Reactions of Isolated Persulfides Provide Insights into the Interplay Between H2S and Persulfide Reactivity.” Free Radic. Biol. Med. 2015, 89, 662-667. [10.1016/j.freeradbiomed.2015.08.017]
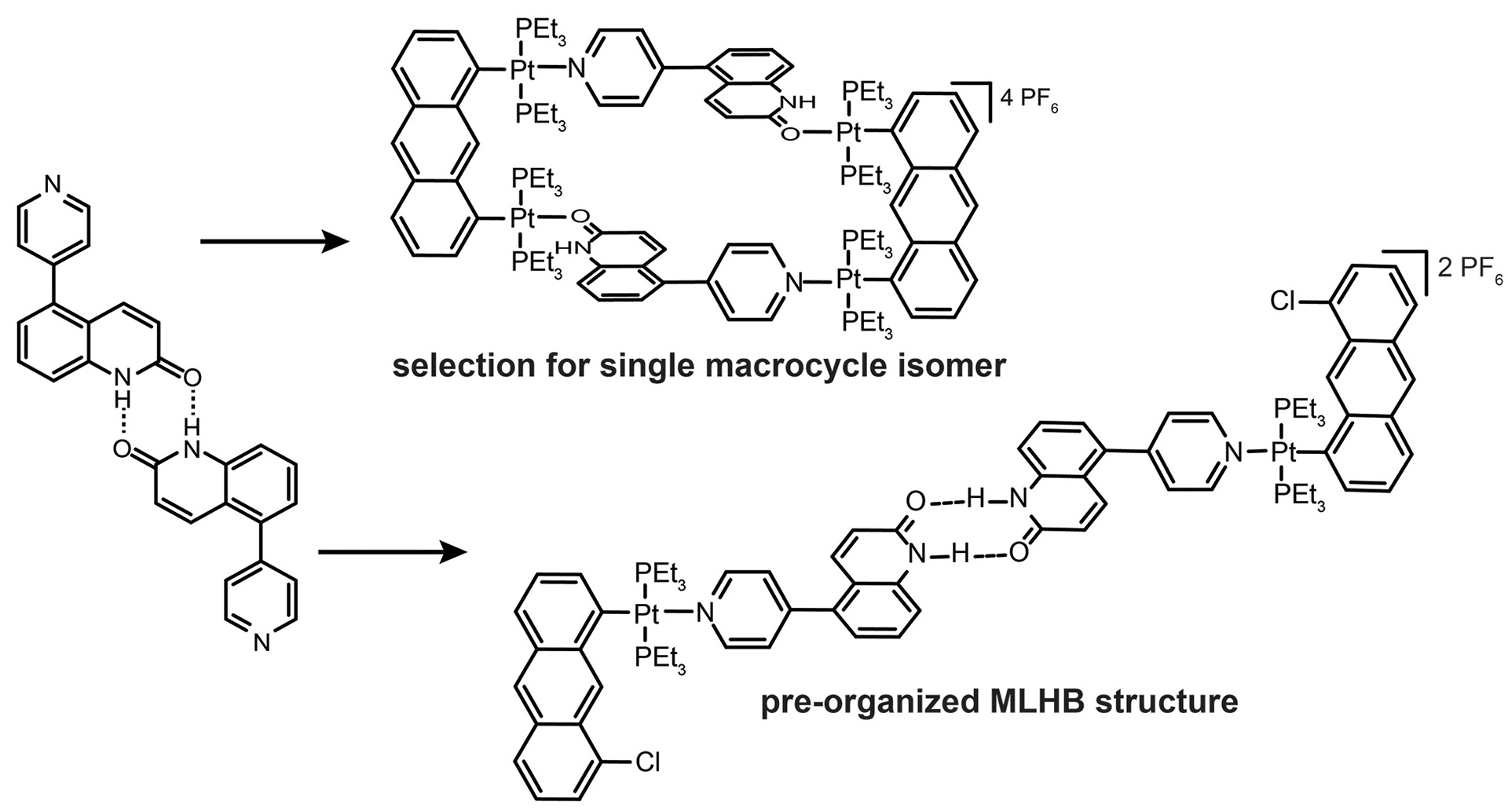
- Sommer, S.K.; Henle, E.A.; Zakharov, L.N.; Pluth, M.D. “Selection for a Single Self-Assembled Macrocycle from a Hybrid Metal-Ligand Hydrogen-Bonded (MLHB) Ligand Subunit” Inorg. Chem. 2015, 54(14), 6910–6916. [10.1021/acs.inorgchem.5b00857]
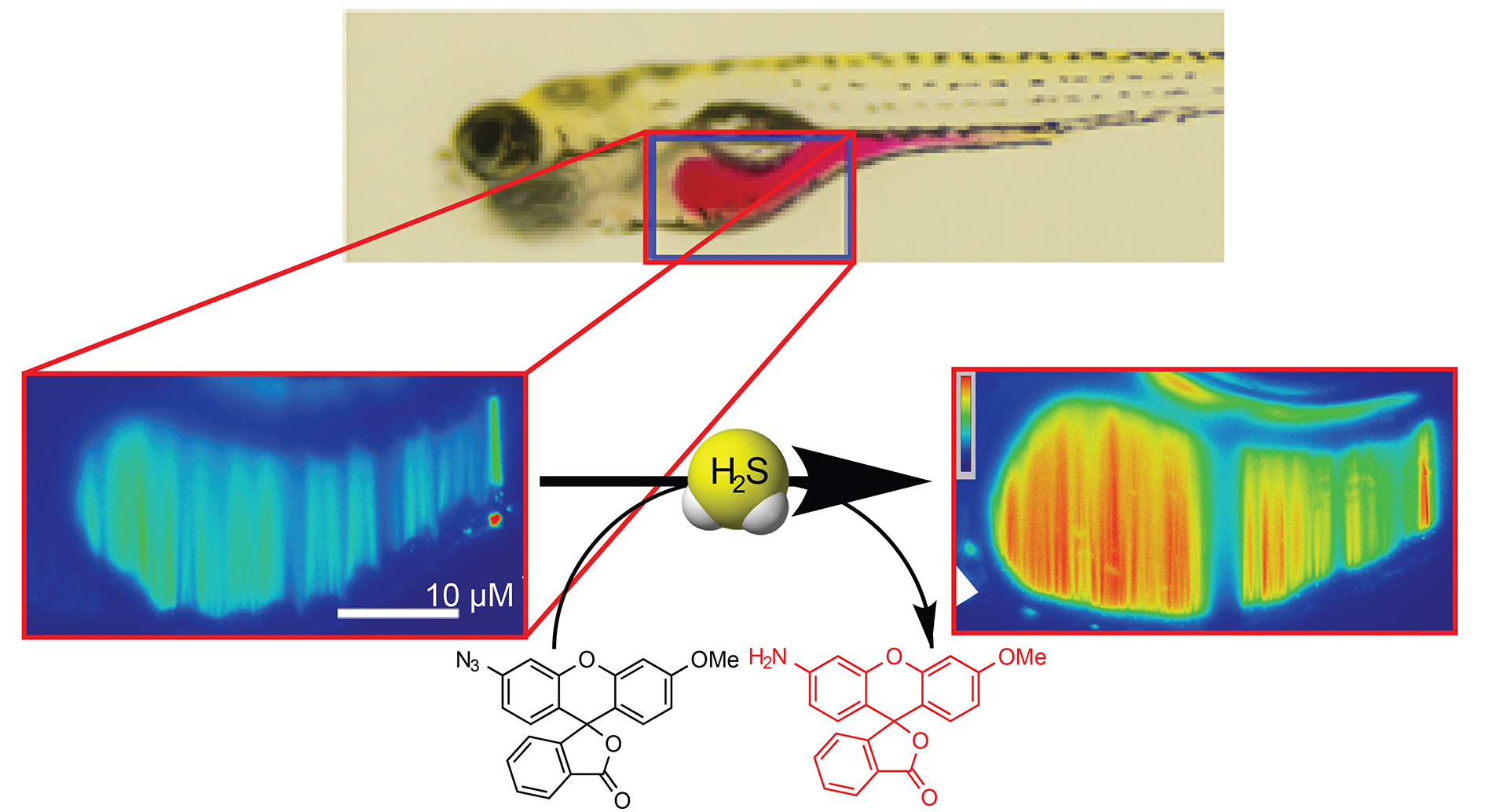
- Hammers, M.D.; Taormina, M.J.; Cerda, M.M.; Montoya, L.A.; Seidenkranz, D.T.; Parthasarathy, R.; Pluth, M.D. “A Bright Fluorescent Probe for H2S Enables Analyte-Responsive, 3D Imaging in Live Zebrafish Using Light Sheet Fluorescence Microscopy.” J. Am. Chem. Soc. 2015, 137(32), 10216-10223. [10.1021/jacs.5b04196]
– JACS Spotlight Article
– Cover article

- Sonke, E.; Verrydt, M.; Postenka, C.O.; Pardhan, S.; Willie, C.J.; Mazzola, C.R.; Hammers, M.D.; Pluth, M.D.; Lobb, I.; Power, N.E.; Chambers, A.F.; Leong, H.S.; Sener, A. “Inhibition of endogenous hydrogen sulfide production in clear-cell renal cell carcinoma cell lines and xenografts restricts their growth, survival and angiogenic potential.” Nitric Oxide 2015, 49(15), 26-39. [10.1016/j.niox.2015.06.001]
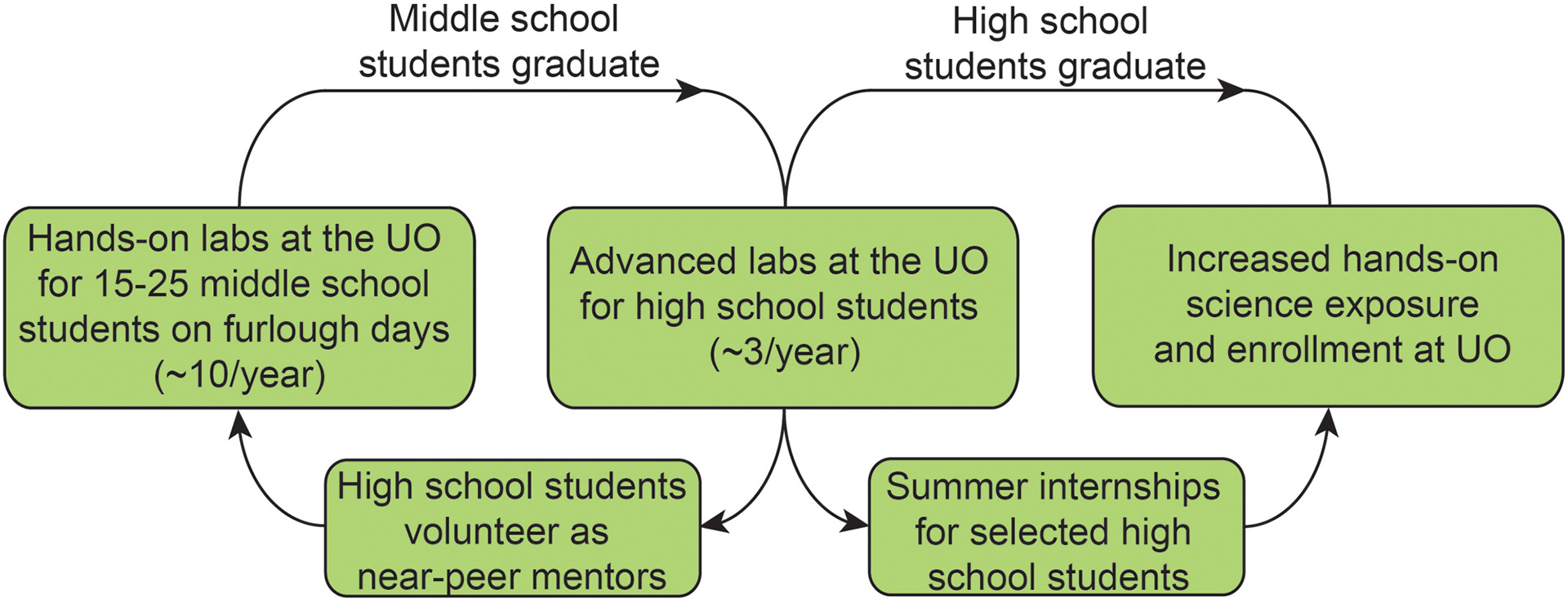
- Pluth, M.D.; Boettcher, S.W.; Nazin, G.V.; Greenaway, A.L.; Hartle, M.D. “Collaboration and Near-Peer Mentoring as a Platform for Sustainable Science Education Outreach.” J. Chem. Educ. 2015, 92(4), 625–630. [10.1021/ed500377m]
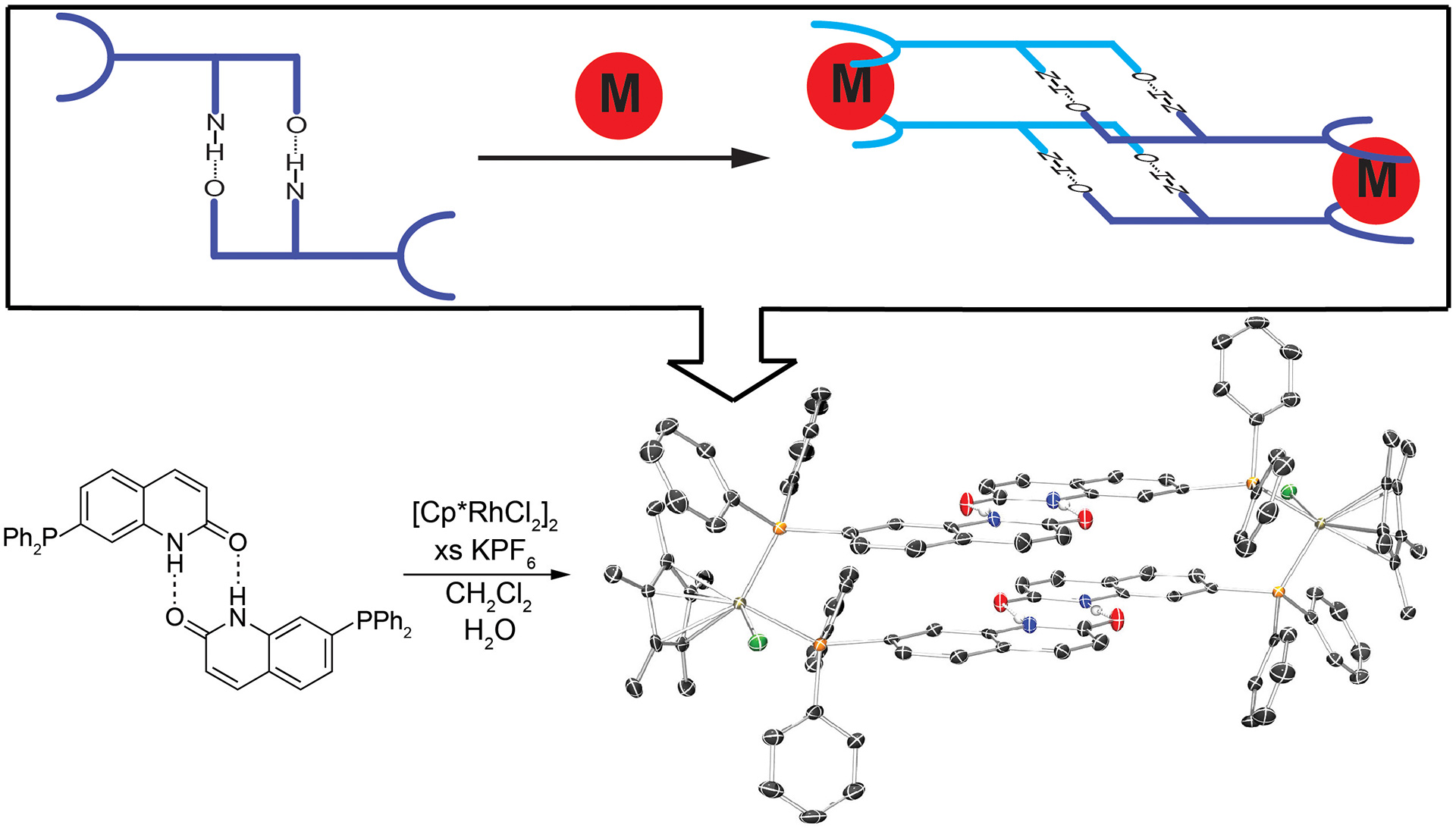
- Sommer, S.K.; Zakharov, L.N.; Pluth, M.D. “Design, Synthesis, and Characterization of Hybrid MetalLigand Hydrogen-Bonded (MLHB) Supramolecular Architectures.” Inorg. Chem. 2015, 54(4), 1912–1918. [10.1021/ic502802f]
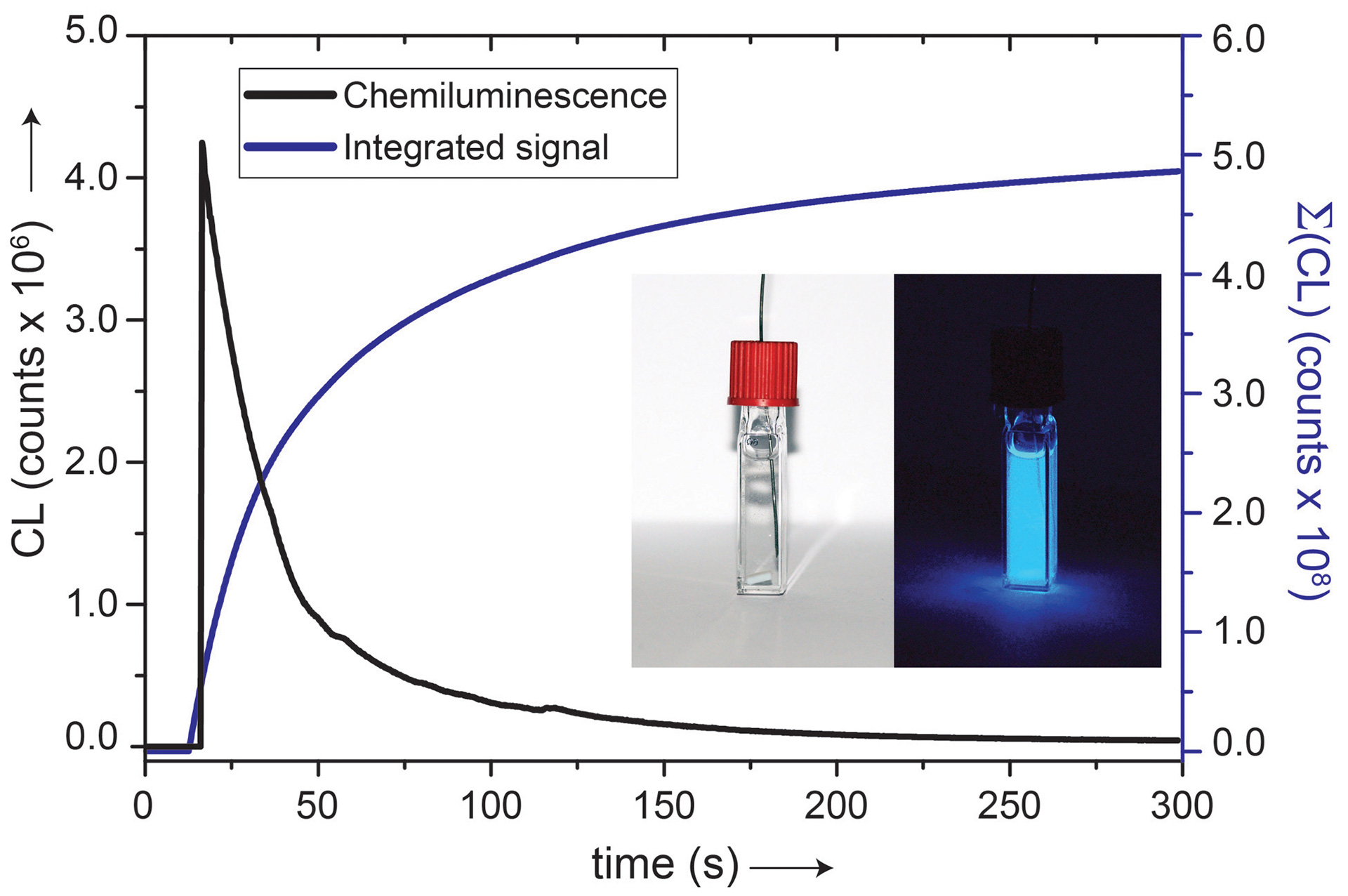
- Bailey, T.S.; Pluth, M.D. “Chemiluminescent Detection of Enzymatically Produced H2S.” Methods Enzymol. 2015, 554, 81-99. [10.1016/bs.mie.2014.11.012]
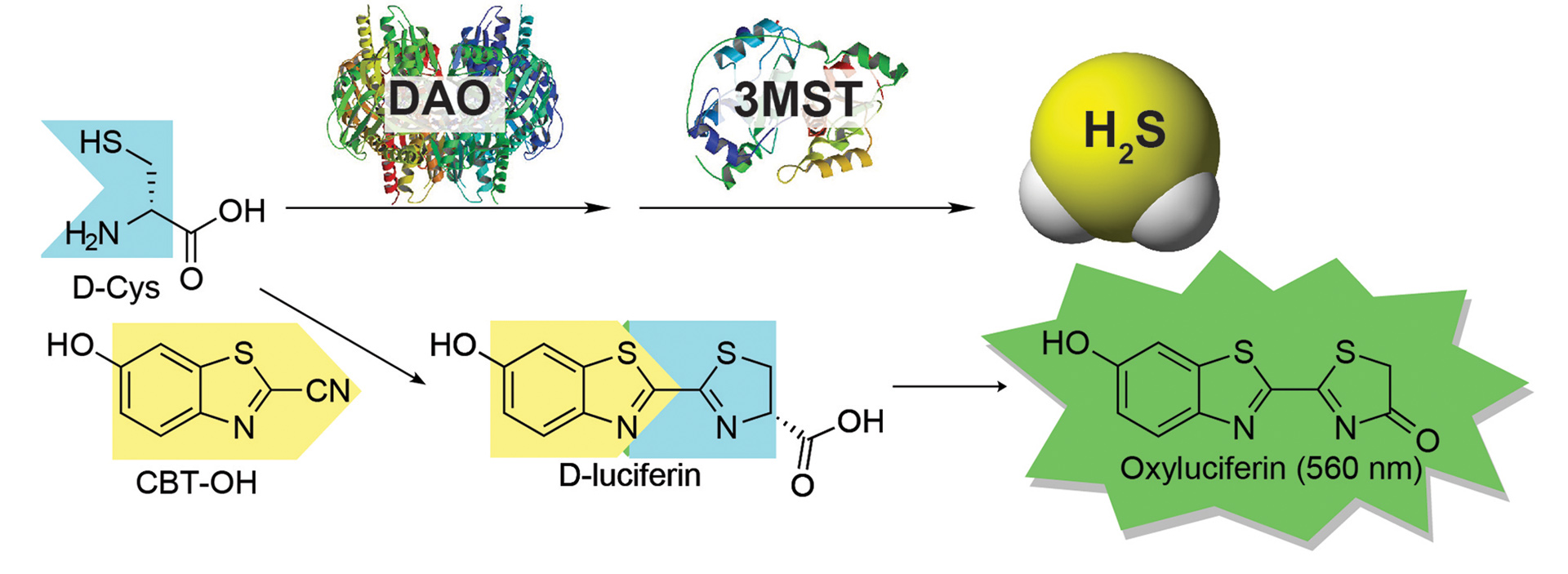
- Bailey, T.S.; Donor, M.T.; Naughton, S.P.; Pluth, M.D. “A Simple Bioluminescent Method for Measuring D-Amino Acid Oxidase Activity.” Chem. Commun. 2015, 51, 5425-5428. [10.1039/c4cc08145e]
- Montoya, L.A.; Shen, X.; McDermott, J.J.; Kevil, C.G.; Pluth, M.D. “Mechanistic Investigations Reveal that Dibromobimane Extrudes Sulfur from Biological Sulfhydryl Sources other than Hydrogen Sulfide.” Chem. Sci. 2015, 6, 294-300. [10.1039/c4sc01875c]
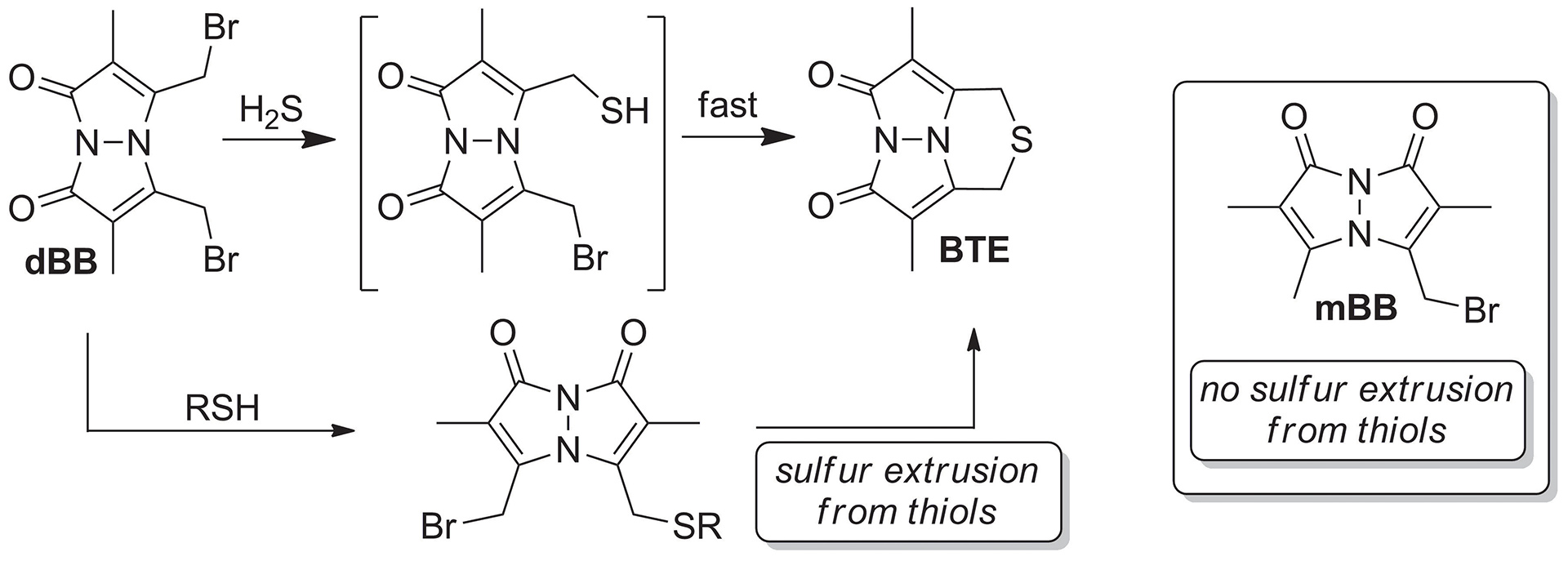
- McGrath, J.M.; Pluth, M.D. “Linear Free Energy Relationships Reveal Structural Changes in Hydrogen-Bonded HostGuest Interactions.” J. Org. Chem. 2014, 79(23), 11797-11801. [10.1021/jo502325w]
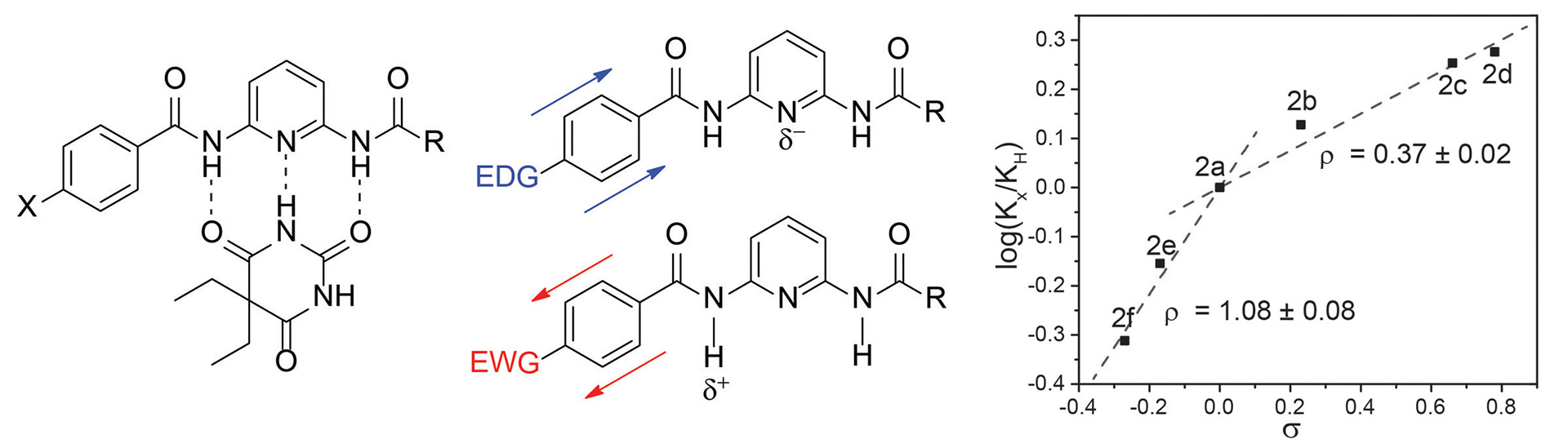
- Bailey, T.S.; Zakharov, L.N.; Pluth, M.D. “Understanding Hydrogen Sulfide Storage: Probing Conditions for Sulfide Release from Hydrodisulfides” J. Am. Chem. Soc. 2014, 136(30), 10573-10576. [10.1021/ja505371z]
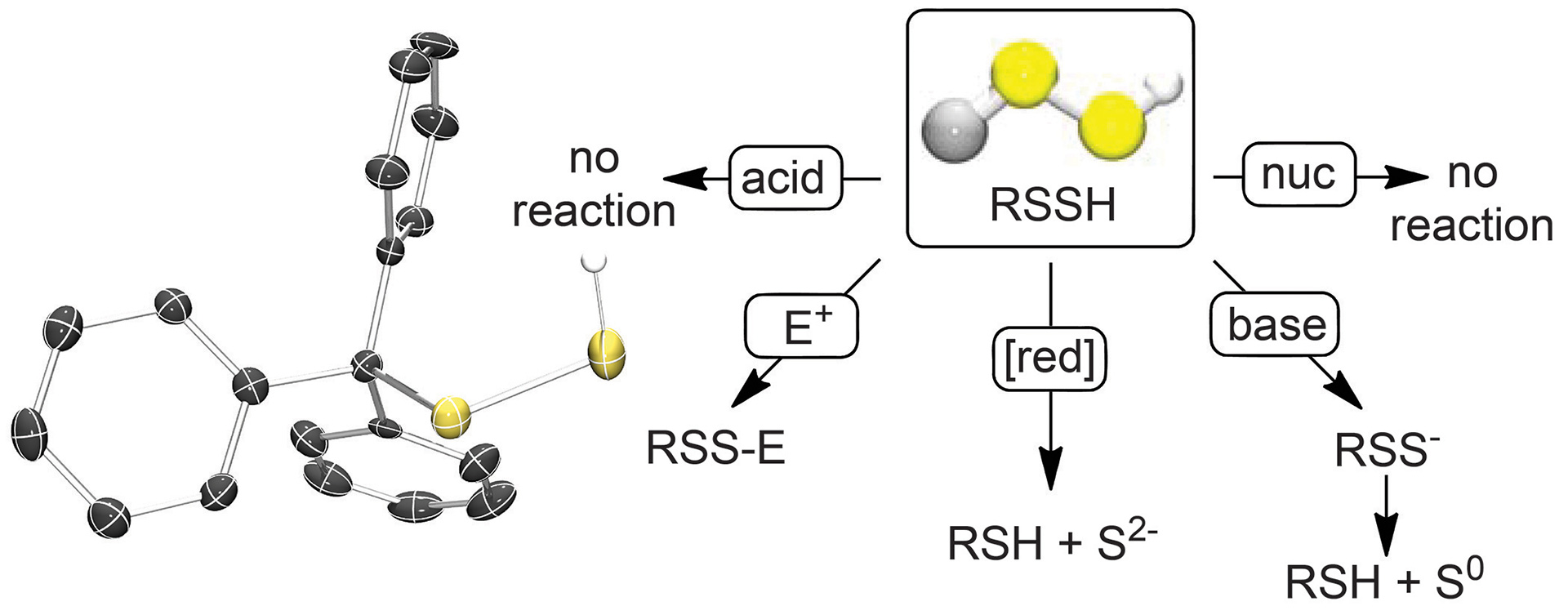
- Hammers, M.D.; Pluth, M.D. “Ratiometric Measurement of Hydrogen Sulfide and Cysteine/Homocysteine Ratios Using a Dual-Fluorophore Fragmentation Strategy” Anal. Chem. 2014, 86(14), 7135–7140.
[10.1021/ac501680d]
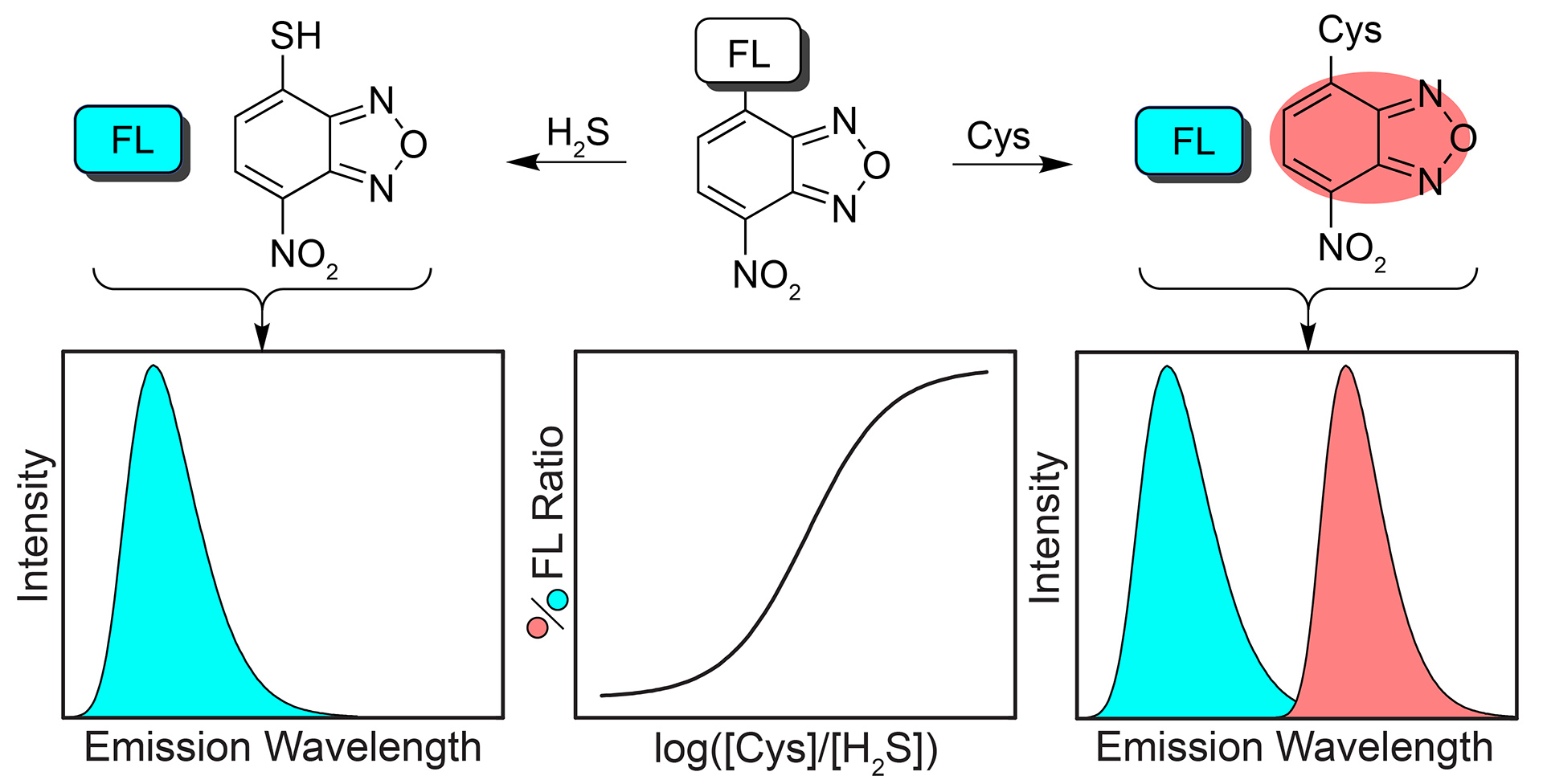
- Montoya, L.A.; Pluth, M.D. “Hydrogen Sulfide Deactivates Common Nitrobenzofurazan-Based Fluorescent Thiol Labeling Reagents.” Anal. Chem. 2014, 86(12), 6032-6039. [10.1021/ac501193r]
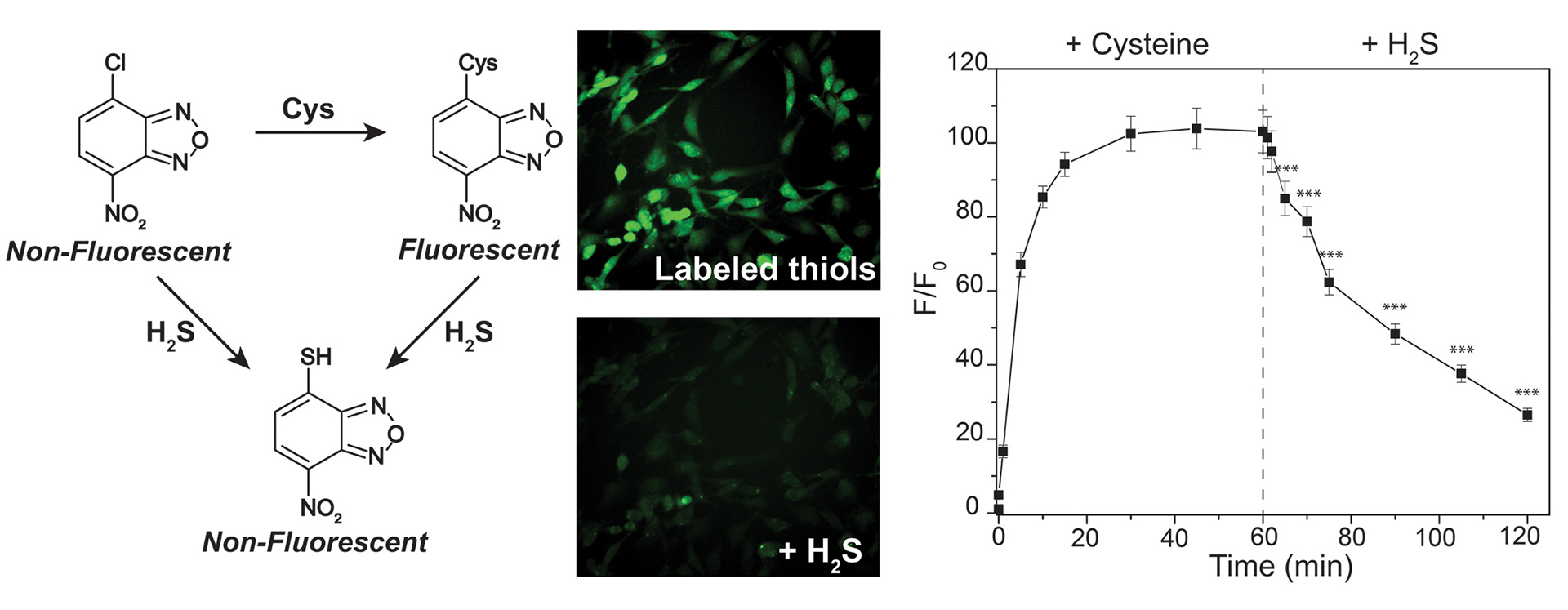
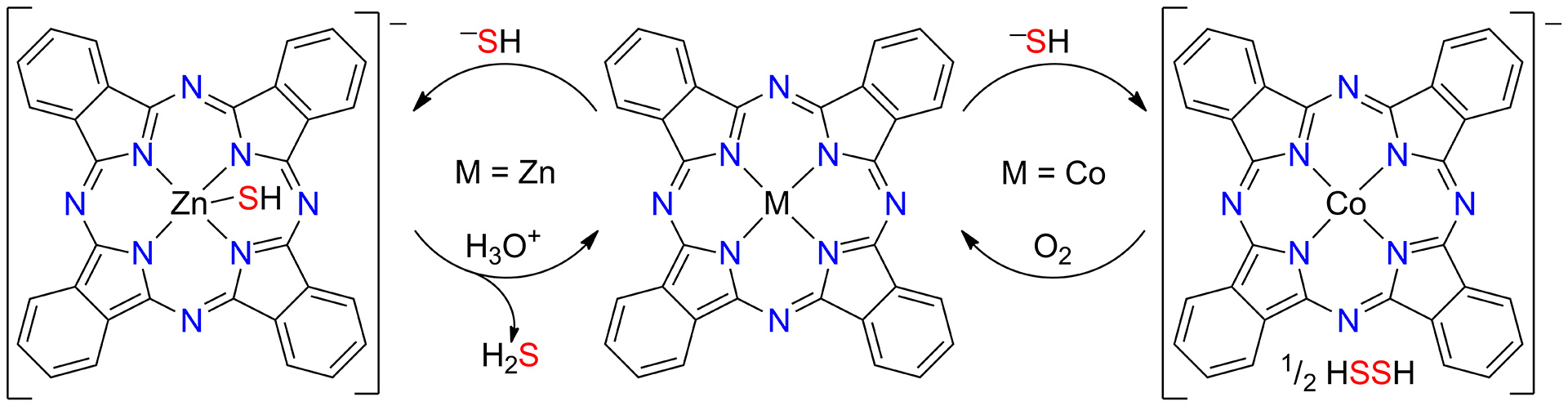
- Hartle, M.D.; Sommer, S.K.; Dietrich, S.R.; Pluth, M.D. “Chemically Reversible Reactions of Hydrogen Sulfide with Metal Phthalocyanines” Inorg. Chem. 2014, 53(15), 7800-7802. [10.1021/ic500664c]
– Cover article

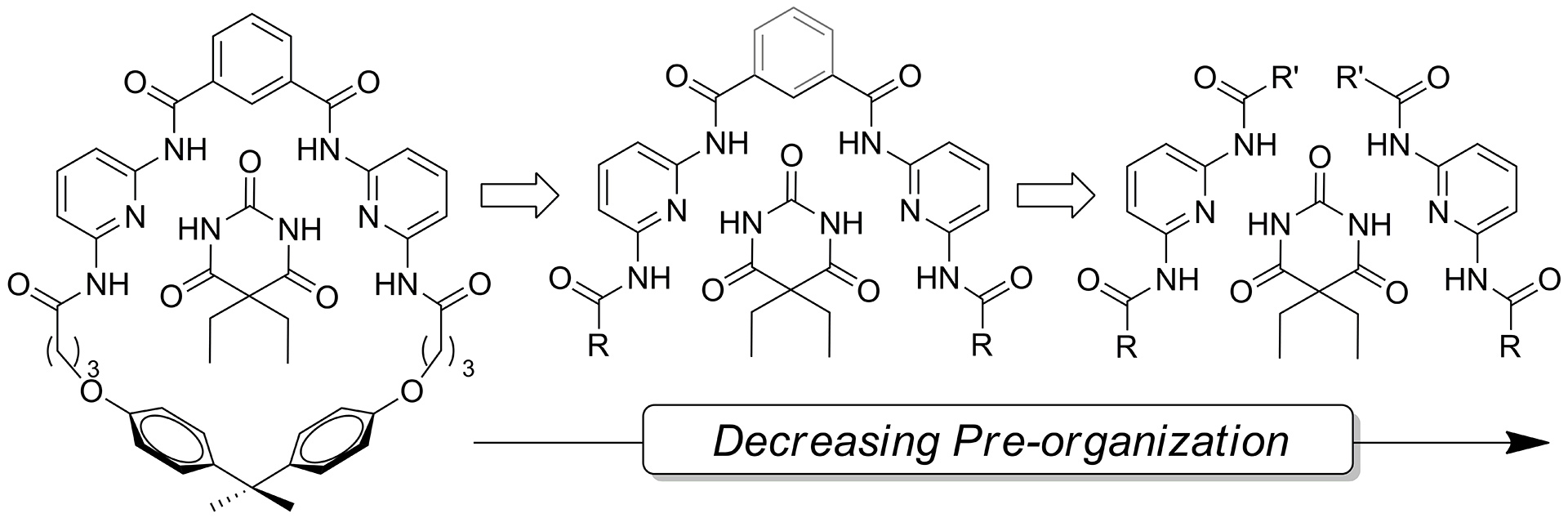
- McGrath, J.M.; Pluth, M.D. “Understanding the Effects of Pre-Organization, Rigidity, and Steric Interactions in Synthetic Barbiturate Receptors” J. Org. Chem. 2014, 79(2), 711-719. [10.1021/jo402500a]
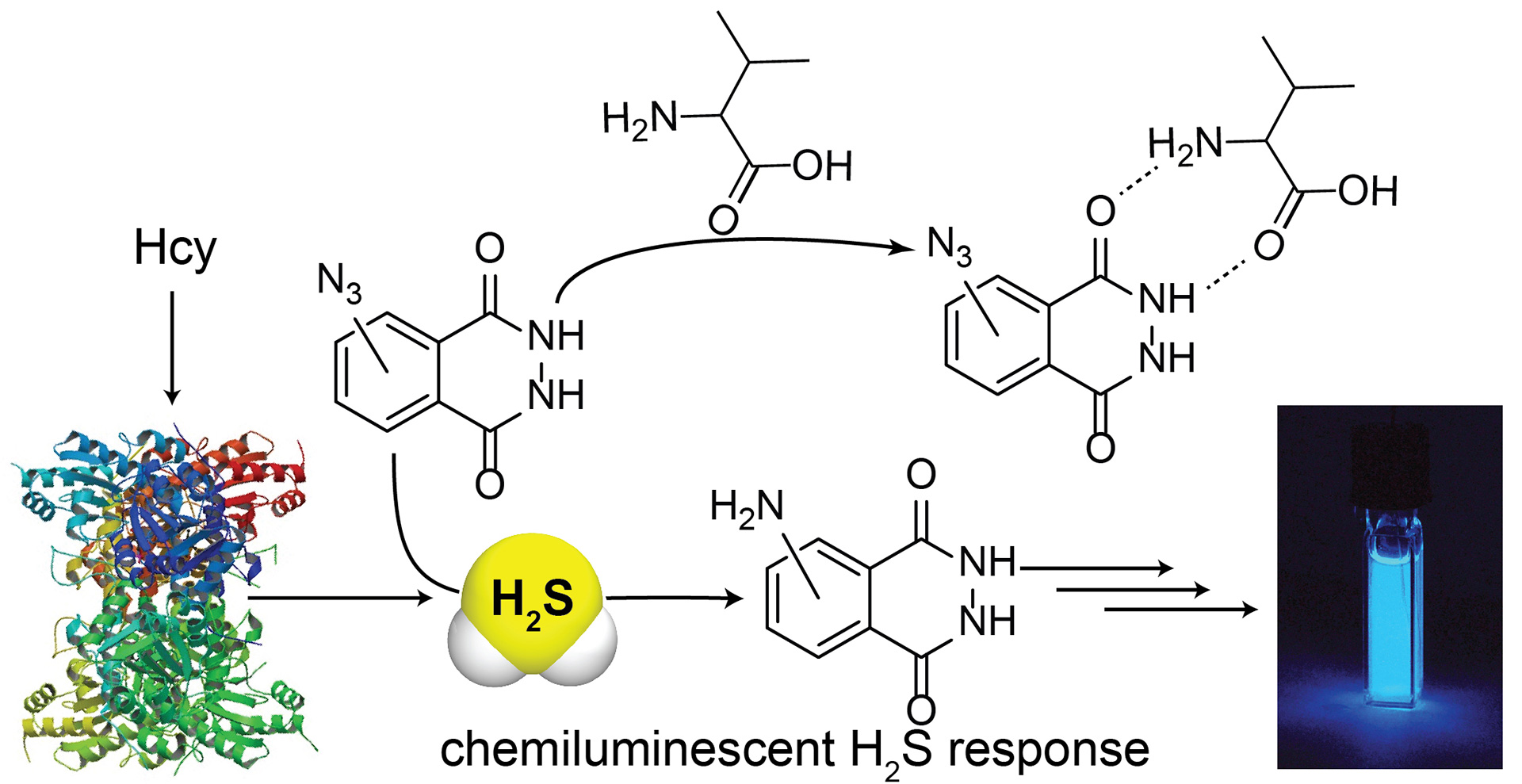
- Bailey, T.S.; Pluth, M.D. “Chemiluminescent Detection of Enzymatically-Produced Hydrogen Sulfide: Substrate Hydrogen Bonding Influences Selectivity for H2S over Biological Thiols” J. Am. Chem. Soc. 2013, 135(44), 16697-16704. [10.1021/ja408909h]
- Pluth, M.D.; Bailey, T.S.; Hammers, M.D.; Montoya, L.A. “Chemical Tools for Studying Biological Hydrogen Sulfide” in Biochalcogen Chemistry: The Biological Chemistry of Sulfur, Selenium, and Tellurium; 2013, 15-32. [pdf]
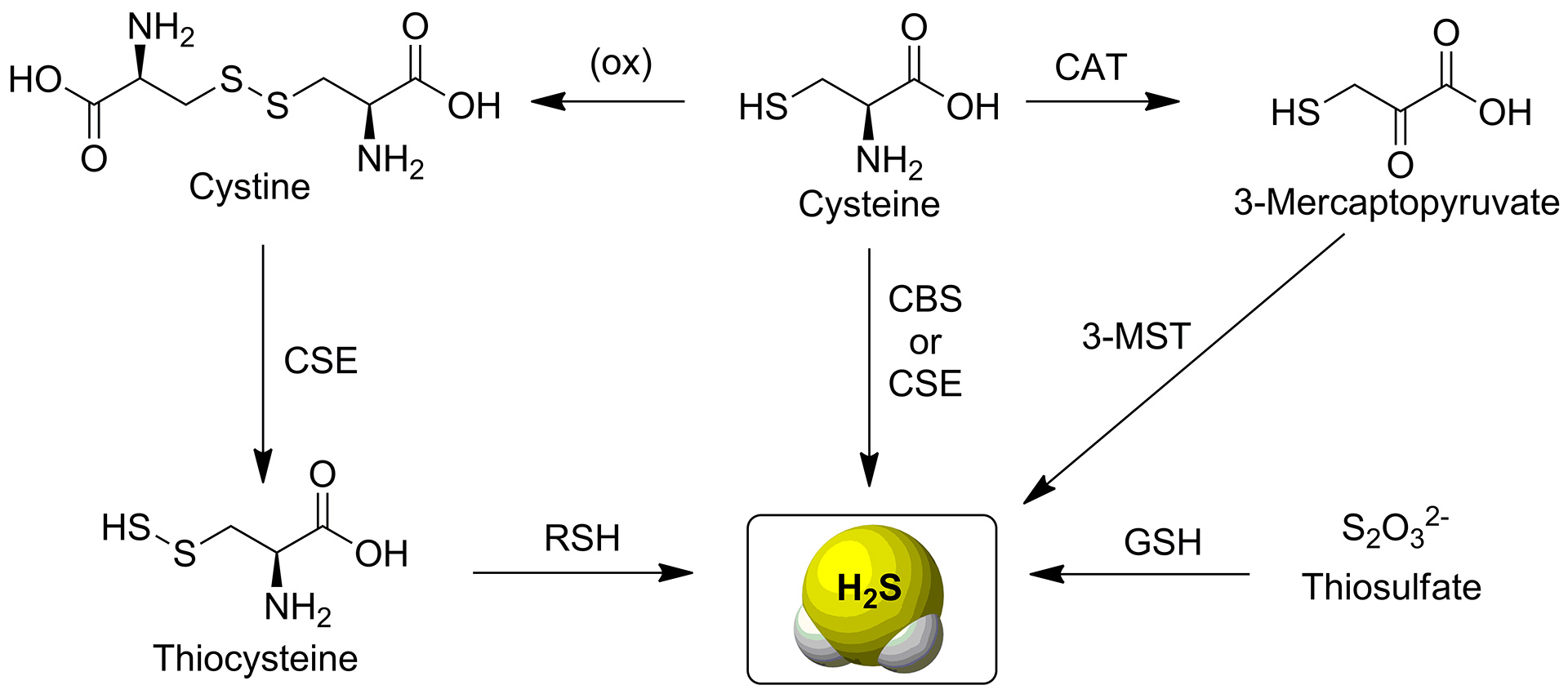
- Montoya, L.A.; Pearce, T.F.; Hansen, R.J.; Zakharov, L.N.; Pluth, M.D. “Development of Selective Colorimetric Probes for Hydrogen Sulfide Based on Nucleophilic Aromatic Substitution” J. Org. Chem. 2013, 78(13), 6550-6557. [10.1021/jo4008095]
– Highlighted by UO Research
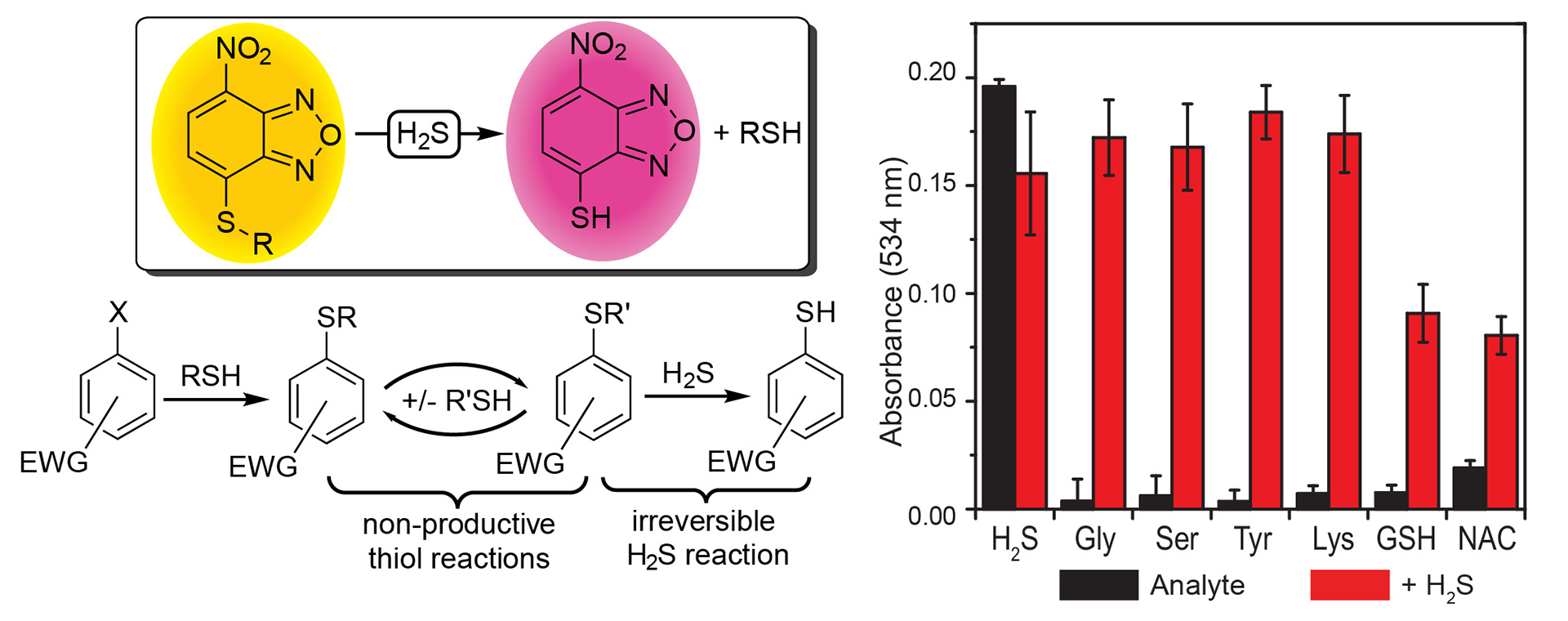
- Montoya, L.A.; Pluth, M.D. “Selective Turn-On Fluorescent Probes for Imaging Hydrogen Sulfide in Living Cells” Chem. Commun. 2012, 48(39), 4767-4769. [10.1039/c2cc30730h]
– One of the top 10 cited papers from Chem. Commun. in 2012
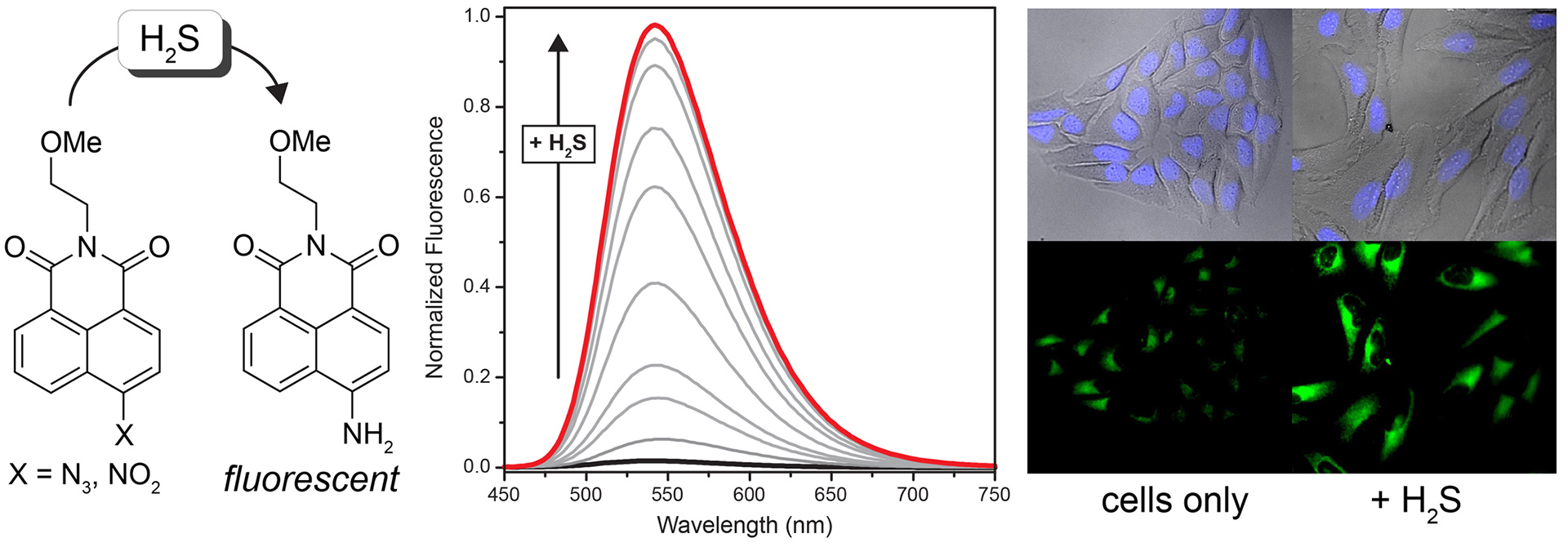
- Chung, C.Y.; Khurana, V.; Aaluck, K.P.; Tardiff, D.F.; Mazzulli, J.R.; Soldner, F.; Baru, V.; Lou, Y.; Freyzon, Y.; Cho, S.; Mungenast, A.E.; Muffat, J.; Mitalipova, M.; Pluth, M.D.; Jui, N.T.; Schule, B.; Lippard, S.J.; Tsai, L.H.; Krainc, D.; Buchwald, S.L.; Jaenisch, R.; Lindquist, S. “Conserved cellular phenotypes and rescue of a-Synuclein toxicity from yeast to Parkinson patient cortical neurons” Science 2013, 342(6161), 983-987. [10.1126/science.1245296]
- Carnes, M.E.; Collins, M.S.; Lindquist, N.R.; Guzman-Percastegui, E.; Pluth, M.D.; Johnson, D.W. “Chloride-catalyzed, multicomponent self-assembly of arsenic thiolates” Chem. Commun. 2014, 50, 73-75. [10.1039/c3cc47093h]
- Ghosh, M.; van den Akker, N.M.S.; Wijnands, K.A.P; Poeze, M.; Weber, C.; McQuade, L.E.; Pluth, M.D; Lippard, S.J; Post, M.J.; Molin, D.G.M; van Zandvoort, M.A.M.J. “Specific visualization of nitric oxide in the vasculature with two-photon microscopy using a copper based fluorescent probe” PLoS One 2013, 8(9), e75331. [10.1371/journal.pone.0075331]
- Pluth, M.D.; Lippard, S.J. “Reversible Binding of Nitric Oxide to an Fe(III) Complex of a Tetra-amido Macrocycle” Chem. Commun. 2012, 48, 11981-11983. [10.1039/c2cc37221e]
- Sartoretto, J.L.; Kalwa, H.; Shiroto, T.; Sartoretto, S.M.; Pluth, M.D.; Lippard, S.J.; Michel, T. “Role of Ca2+ in the Control of H2O2-Modulated Phosphorylation Pathways Leading to eNOS Activation in Cardiac Myocytes” PLoS One 2012, 7(9), e44627. [10.1371/journal.pone.0044627]
- Sartoretto, J.L.; Kalwa, H.; Pluth, M.D.; Lippard, S.J.; Michel, T. “Hydrogen peroxide differentially modulates cardiac myocyte nitric oxide synthesis” Proc. Natl. Acad. Sci. USA 2011, 108(38), 15792-15797. [10.1073/pnas.1111331108]
- Pluth, M.D.; Chan, M.R.; McQuade, L.E.; Lippard, S.J. “Seminaphthofluorescein-Based Fluorescent Probes for Imaging Nitric Oxide in Live Cells” Inorg. Chem. 2011, 50(19), 9385-9392. [10.1021/ic200986v]
- Pluth, M.D.; Tomat, E.; Lippard, S.J. “Biochemistry of Mobile Zinc and Nitric Oxide Revealed by Fluorescent Sensors” Annu. Rev. Biochem. 2011, 80, 333-355. [10.1146/annurev-biochem-061009-091643]
- McQuade, L.E.; Pluth, M.D.; Lippard, S.J. “Mechanism of Nitric Oxide Reactivity and Fluorescence Enhancement of the NO-Specific Probe, CuFL1” Inorg. Chem. 2010, 49(17), 8025-8033. [10.1021/ic101054u]
- Hastings, C.J.; Pluth, M.D.; Bergman, R.G.; Raymond, K.N. “Enzyme-Like Catalysis of the Nazarov Cyclization by Supramolecular Encapsulation” J. Am. Chem. Soc. 2010, 132(20), 6938-6940. [10.1021/ja102633e]
- Pluth, M.D.; McQuade, L.E.; Lippard, S.J. “Cell-Trappable Fluorescent Probes for Nitric Oxide Visualization in Living Cells” Org. Lett. 2010, 12(10), 2318-2321. [10.1021/ol1006289]
- Sgarlata, C.; Mugridge, J.S.; Pluth, M.D.; Tiedemann, B.E.F.; Zito, V.; Arena, G.; Raymond, K.N. “External and Internal Guest Binding of a Highly Charged Supramolecular Host in Water: Deconvoluting the Very Different Thermodynamics” J. Am. Chem. Soc. 2010, 132(3), 1005-1009. [10.1021/ja9056739]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. “Proton Mediated Chemistry and Catalysis in a Self-Assembled Supramolecular Host” Acc. Chem. Res. 2009, 42(10), 1650-1659. [10.1021/ar900118t]
- Pluth, M.D.; Fiedler, D.; Mugridge, J.S.; Bergman, R.G.; Raymond, K.N. “Encapsulation and Characterization of Proton-Bound Amine Homodimers in a Water Soluble, Self-Assembled Supramolecular Host” Proc. Nat. Acad. Sci. USA 2009, 106, 10438-10443. [10.1073/pnas.0809806106]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. “The Acid Hydrolysis Mechanism of Acetals Catalyzed by a Supramolecular Assembly in Basic Solution” J. Org. Chem. 2009, 74(1), 58-63. [10.1021/jo802131v]
– Selected by journal as Featured Article - Pluth, M.D.; Johnson, D.W.; Szigethy, G.; Davis, A.V.; Teat, S.J.; Oliver, A.G.; Bergman, R.G.; Raymond, K.N. “Structural Consequences of Anionic Host–Cationic Guest Interactions in a Supramolecular Assembly” Inorg. Chem. 2009, 48(1), 111-120. [10.1021/ic8012848]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. “Supramolecular Catalysis of Orthoformate Hydrolysis in Basic Solution: An Enzyme-Like Mechanism” J. Am. Chem. Soc. 2008, 130(34), 11423-11429. [10.1021/ja802839v]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. “Acceleration of Amide Bond Rotation by Encapsulation in the Hydrophobic Interior of a Water-Soluble Supramolecular Assembly” J. Org. Chem. 2008, 73(18), 7132-7136. [10.1021/jo800991g]
- Hastings, C.J.; Pluth, M.D.; Biros, S.M.; Bergman, R.G.; Raymond, K.N. “Simultaneously Bound Guests and Chiral Recognition: A Chiral Self-Assembled Supramolecular Host Encapsulates Hydrophobic Guests” Tetrahedron 2008, 64(36), 8362-8367. [10.1016/j.tet.2008.05.131]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. “Encapsulation of Protonated Diamines in a Water-Soluble Chiral Supramolecular Assembly Allows for Measurement of Hydrogen-Bond Breaking Followed by Nitrogen Inversion/Rotation (NIR)” J. Am. Chem. Soc. 2008, 30(20), 6362-6366. [10.1021/ja076691h]
- Pluth, M.D.; Tiedemann, B.E.F.; van Halbeek, H.; Nunlist, R.; Raymond, K.N. “Diffusion of a Highly-Charged Supramolecular Assembly: Direct Observation of Ion-Association in Water” Inorg. Chem. 2008, 47(5), 1411-1413. [10.1021/ic7020885]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. “Selective Organic and Organometallic Reactions in Water-Soluble Host-Guest Supramolecular Systems” The Nucleus 2008, 8, 10-17, 20-21. [pdf]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. “Selective Stoichiometric and Catalytic Reactivity in the Confines of a Chiral Supramolecular Assembly” in Supramolecular Catalysis; van Leeuwen, P. W. N. M, Ed.; Wiley-VCH Germany, 2008, pp 165-197. [10.1002/9783527621781.ch7]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. “Catalytic Deprotection of Acetals in Basic Solution Using a Self-Assembled Supramolecular ‘Nanozyme'” Angew. Chem. Int. Ed. 2007, 119(45), 8741-8743. [10.1002/anie.200703371]
– Selected by journal as VIP (Very Important Paper) article
– Highlighted at http://www.organic-chemistry.org, March 31, 2008 - Nolin, K.A.; Krumper, J.R.; Pluth, M.D.; Bergman, R.G.; Toste, F.D. “Analysis of an Unprecedented Mechanism for the Catalytic Hydrosilylation of Carbonyl Compounds” J. Am. Chem. Soc. 2007, 129(47), 14684-14696. [10.1021/ja074477n]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. “Making Amines Strong Bases: Thermodynamic Stabilization of Protonated Guests in a Highly-Charged Supramolecular Host” J. Am. Chem. Soc. 2007, 129(37), 11459-11467. [10.1021/ja072654e]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. “Acid Catalysis in Basic Solution: A Supramolecular Host Promotes Orthoformate Hydrolysis” Science 2007, 316(5821), 85-88. [10.1126/science.1138748]
– Subject of Chemical & Engineering News concentrate: 2007, 85(15), 36. - Pluth, M.D.; Raymond, K.N. “Reversible Guest Exchange Mechanisms in Supramolecular Host-Guest Assemblies” Chem. Soc. Rev. 2007, 36(2), 161-171. [10.1039/b603168b]
- Seitz, M.; Pluth, M.D.; Raymond, K.N. “1,2-HOIQO – A Highly Versatile 1,2-HOPO Analog” Inorg. Chem. 2007, 46(2), 351-353. [10.1021/ic0614869]
- Davenport, T.C.; Gleason, A.E.; Liska, P.J.; Mugridge, J.S.; Pluth, M.D. “N,N’-(Pyrene-1,8-diyl)bis(2,3-dimethoxybenzaldehyde)” Acta Cryst. E. 2007, E63(8), 3621-3622. [10.1107/s1600536807035751]
- Breno, K.L.; Ahmed, T.J.; Pluth, M.D.; Balzarek, C.; Tyler, D.R. “Organometallic Chemistry in Aqueous Solution: Reactions Catalyzed by Water-Soluble Molybdocenes” Coord. Chem. Rev. 2006, 250(9-10), 1141-1151. [10.1016/j.ccr.2005.12.001]
- Demoin, D.W.; Pluth, M.; Soo, H.S.; Xu, Y. “Dimethoxyphosphinoyl Phenyl Ketone p-Tolylsulfonylhydrazone” Acta Cryst. E.2006, E62(8), 3551-3552. [10.1107/s1600536806025451]
- Pluth, M.D.; Bergman, R.G.; Raymond, K.N. “Encapsulation of Cationic Organometallic Guests by a Chiral Self-Assembled Supramolecular Cage: Enantioselective Binding, Dynamic Resolution, and Selective C-H Bond Activation” Chemtracts 2004, 17, 515-522. [link]
- Breno, K.L.; Pluth, M.D.; Landorf, C.W.; Tyler, D.R. “Aqueous Phase Organometallic Catalysis Using (MeCp)2Mo(OH)(H2O)+Intramolecular Attack of Hydroxide on Organic Substrates” Organometallics 2004, 23(8), 1738-1746. [10.1021/om0342558]
- Breno, K.L.; Pluth, M.D.; Tyler, D.R. “Organometallic Chemistry in Aqueous Solution. Hydration of Nitriles to Amides Catalyzed by a Water-Soluble Molybdocene, (MeCp)2Mo(OH)(H2O)+” Organometallics 2003, 22(6), 1203-1211. [10.1021/om020845e]
- Synthetic reporters for hydrosulfide. Haley, M.M.; Pluth, M.D.; Johnson, D.W. US Patent No. 11,187,690; Issued 11/30/2021. [link]
- Compound embodiments that release H2S by reaction with a reactive compound and methods of making and using the same. Pluth, M.D.; Zhao, Y. Cerda, M.M. US Patent No. 11,078,157; Issued 8/3/2021 [link]
- Compound embodiments for hydrogen sulfide production and methods of making and using the same. US Patent No. 11,040,942; Issued 6/22/2021. Pluth, M.D.; Zhao, Y. [link]
- Compounds for Carbonyl Sulfide/Carbon Disulfide/Hydrogen Sulfide Release and Methods of Making and Using the Same. US Patent No. 10,725,055; Issued 7/28/2020. Pluth, M.D.; Steiger, A.K.; Zhao, Y. [link]
- Compounds for Determining the Presence of Hydrogen Sulfide and Methods of Use. US Patent No. 9,664,696; Issued 5/30/2017. Pluth, M.D.; Montoya, L.A.; Bailey, T.S. Pearce, T.F. [link]