Outreach
Middle- and High-School Outreach Activities
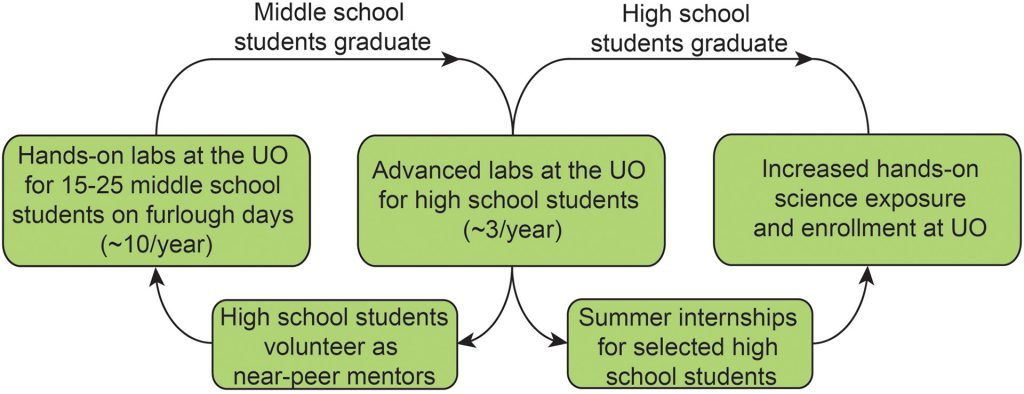
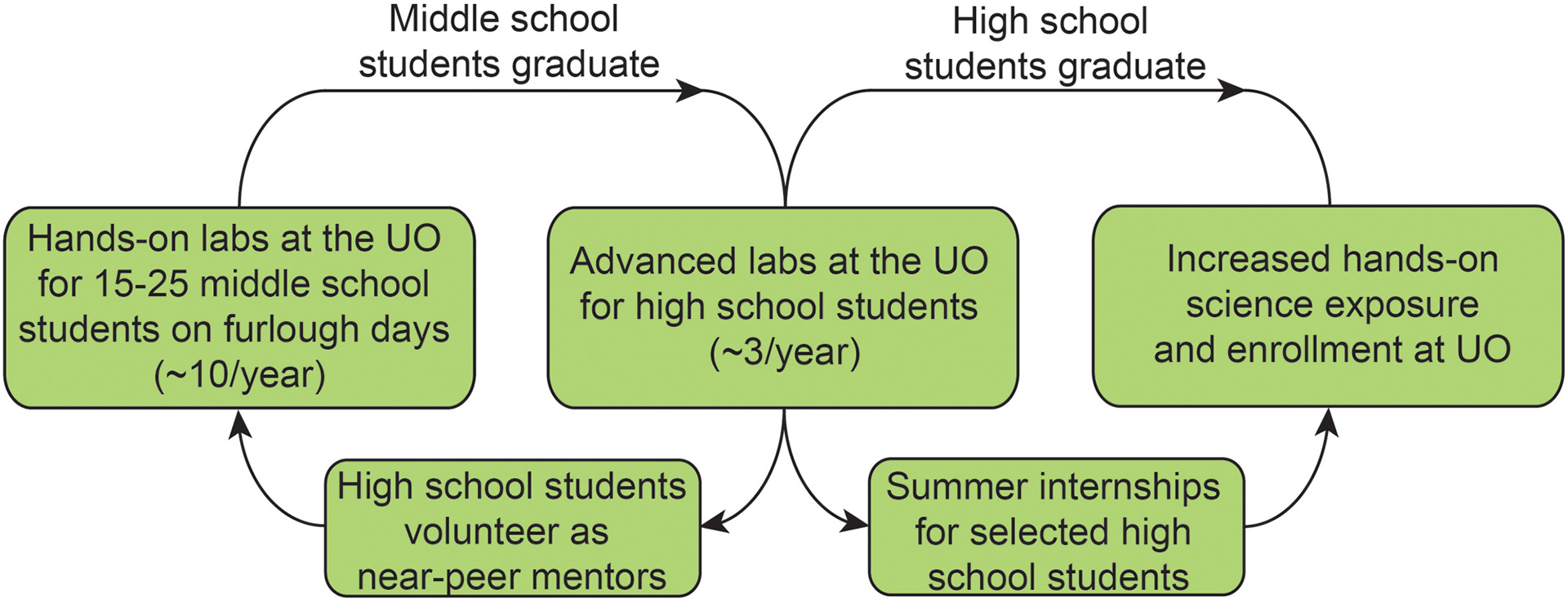
In collaboration with the Boettcher and Nazin groups, we have developed a middle- and high-school outreach program with Springfield School District to supplement science education. Initially designed to bring local student to the UO to run science experiments on days in which school was cancelled due to budget cuts, the ‘Furlough-Friday / Mad Duck‘ science program has evolved to now regularly provide hands-on science education to local students.
Key components of the program include:
- Laboratory-based learning activities for Hamlin Middle School students, who come to the UO campus to participate in hands-on science outreach activities. The activities are run by UO faculty and graduate students, along with Springfield High School near-peer volunteers.
- Classes of High School chemistry students from Springfield High School perform advanced chemistry experiments either in UO teaching or faculty labs using state-of-the-art equipment.
- Development and curation of modular laboratory activities for middle- and high-school students.
Highlighting the success of the program, we run ~10 outreach events per year, each with 10-20 students coming the UO to participate in hands-on science activities during the first 5 years of the program. In addition, this program has been covered by UO, local, and national media outlets, including Chem. & Eng. News.


A summary of the program design and implementation was published Open Access in J. Chem. Educ.
Pluth, M.D.; Boettcher, S.W.; Nazin, G.V.; Greenaway, A.L.; Hartle, M.D. “Collaboration and Near-Peer Mentoring as a Platform for Sustainable Science Education Outreach.” J. Chem. Educ. 2015, 92(4), 625–630. [10.1021/ed500377m]
These activities have been supported financially, the the Camille and Henry Dreyfus Foundation, broader impacts from the National Science Foundation, and local sponsors including LTD, TrackTown Pizza, and the Springfield School District.
Open-Access Publications to Enhance Accessibility
In an effort to increase the accessibility of multidisciplinary research from our group, a number of our papers have been published in freely-accessible open-access formats. These include:
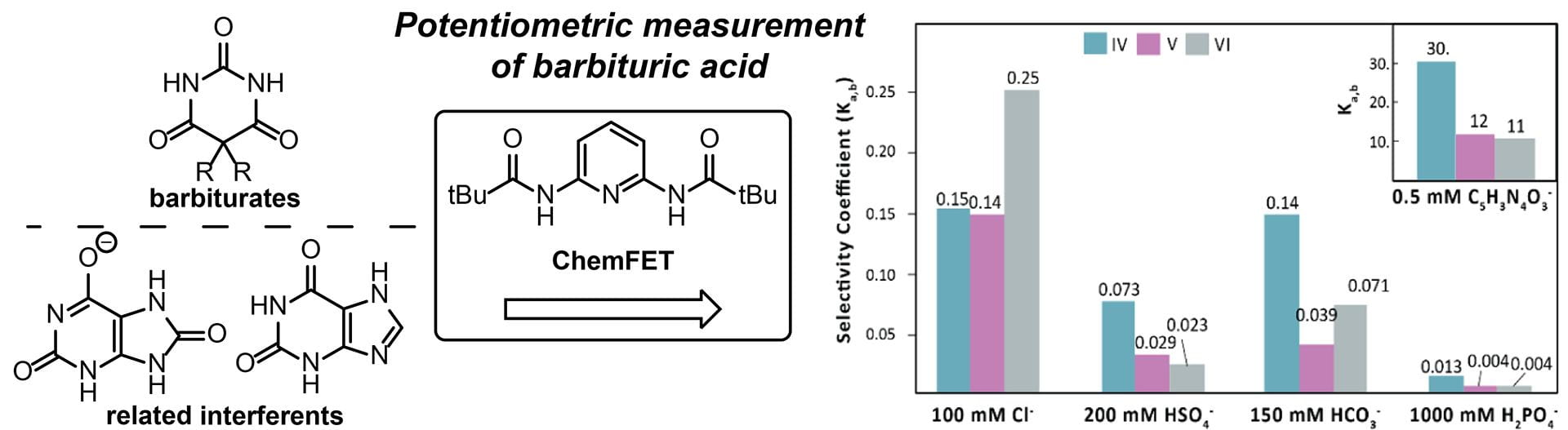
OA29. Kuhl, G.M.; Seidenkranz, D.T.; Pluth, M.D.; Johnson, D.W.; Fontenot, S.A. “Potentiometric Measurement of Barbituric Acid by Integration of Supramolecular Receptors into ChemFETs.” Sens. Bio-Sens. Res., 2021, 100397. [10.1016/j.sbsr.2021.100397]
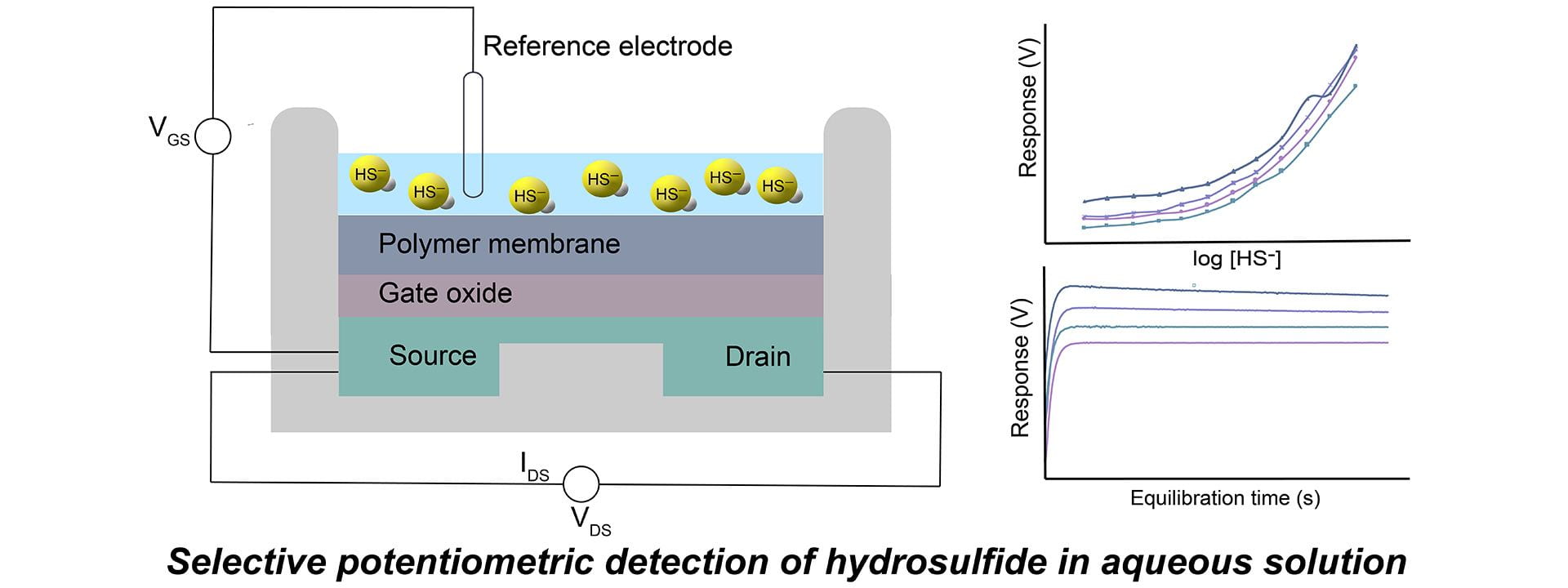
OA28. Sherbow, T.J.; Kuhl, G.M.; Lindquist, G.A.; Levine, J.D.; Pluth, M.D.; Johnson, D.W.; Fontenot, S.A. “Hydrosulfide-Selective ChemFETs for Aqueous H2S/HS− Measurement.” Sens. Bio-Sens. Res., 2021, 100394. [10.1016/j.sbsr.2020.100394]
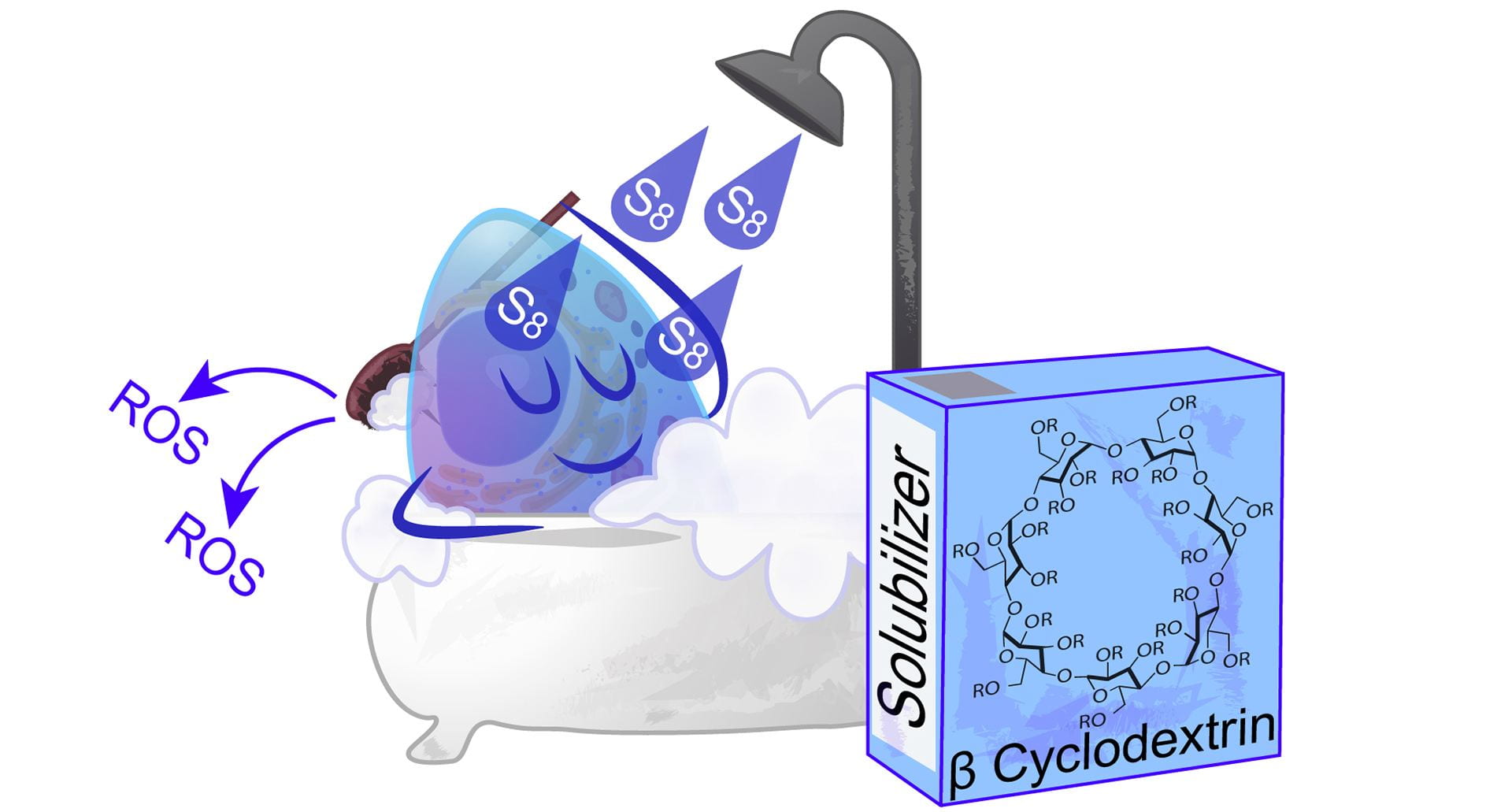
OA27. Bolton, S.G.; Pluth, M.D. “Modified Cyclodextrins Solubilize Elemental Sulfur in Water and Enable Biological Sulfane Sulfur Delivery.” Chem. Sci. 2020, 11, 11777-11784. [10.1039/D0SC04137H]
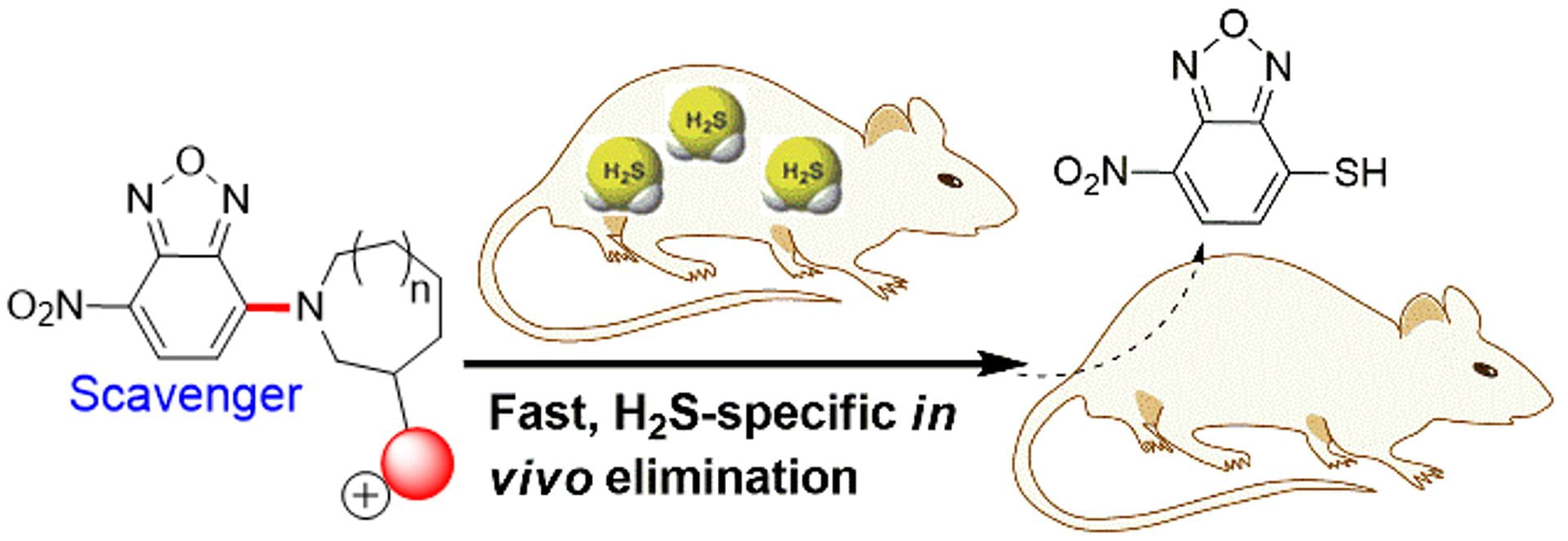
OA26. Ismail, I.; Chen, Z.; Sun, L.; Ji, X.; Ye, H.; Kang, X.; Huang, H.; Song, H.; Bolton, S.G.; Xi, Z.; Pluth, M.D.; Long, Y. “Highly Efficient H2S Scavengers via Thiolysis of Positively-Charged NBD Amines” Chem. Sci. 2020, 11, 7823-7828. [10.1039/D0SC01518K]
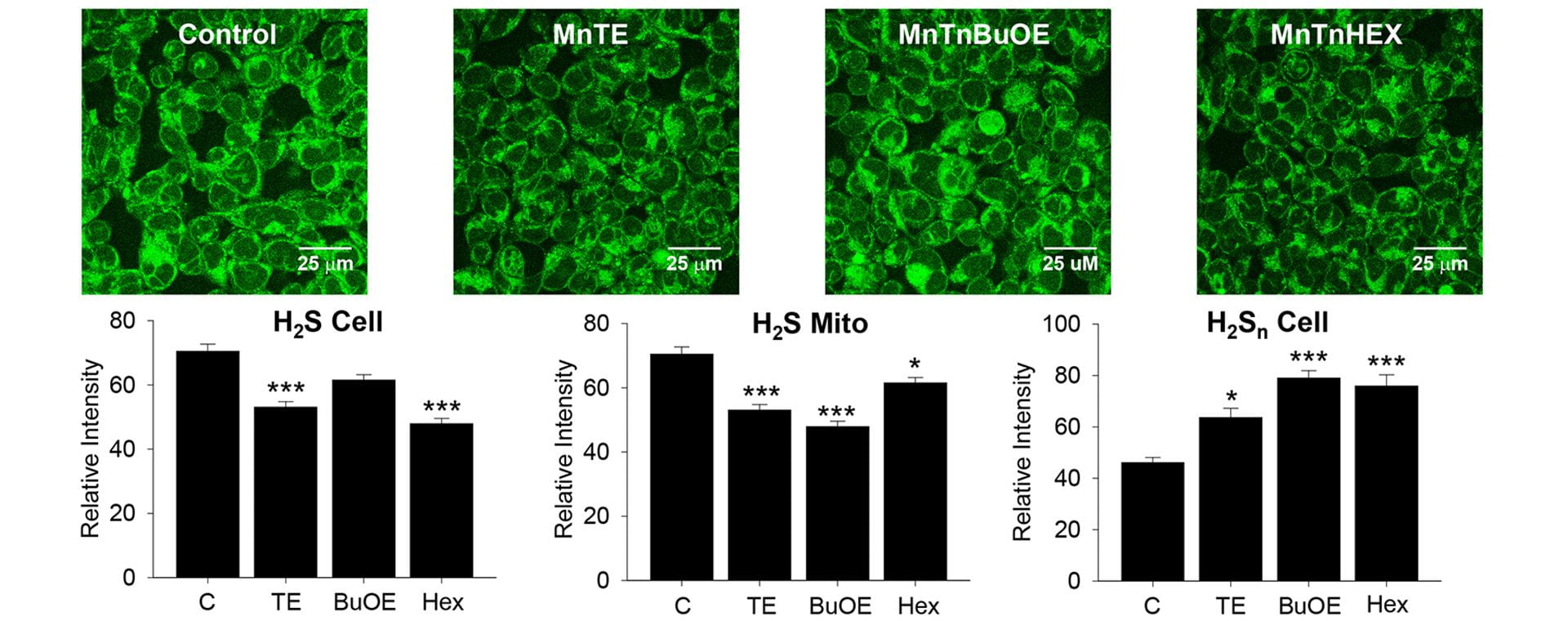
OA25. Olson, K.R.; Gao, Y.; Steiger, A.K.; Pluth, M.D.; Tessier, C.R.; Markel, T.A.; Boone, D.; Stahelin, R.V.; Batinic-Haberle, I.; Straubg, K.D. “Effects of Manganese Porphyrins on Cellular Sulfur Metabolism.” Molecules. 2020, 25(4), 980. [10.3390/molecules25040980]
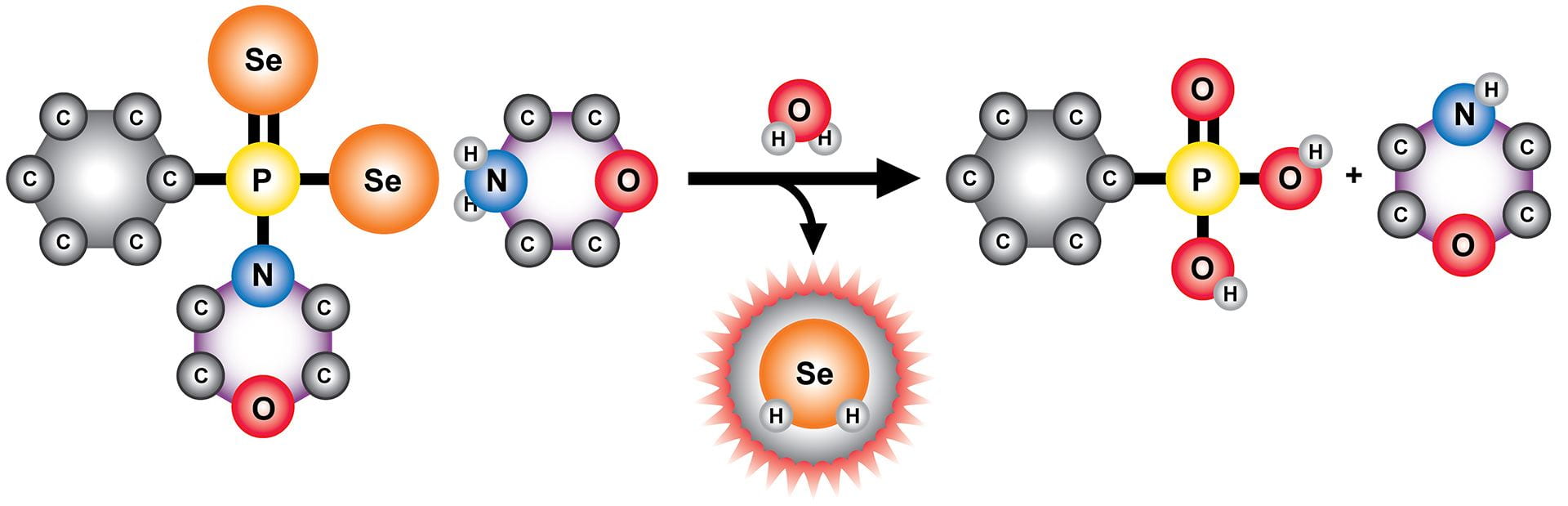
OA24. Newton, T.D.; Pluth, M.D. “Development of a Hydrolysis-Based Small-Molecule Hydrogen Selenide (H2Se) Donor” Chem. Sci. 2019, 10, 10723-10727. [10.1039/C9SC04616J]
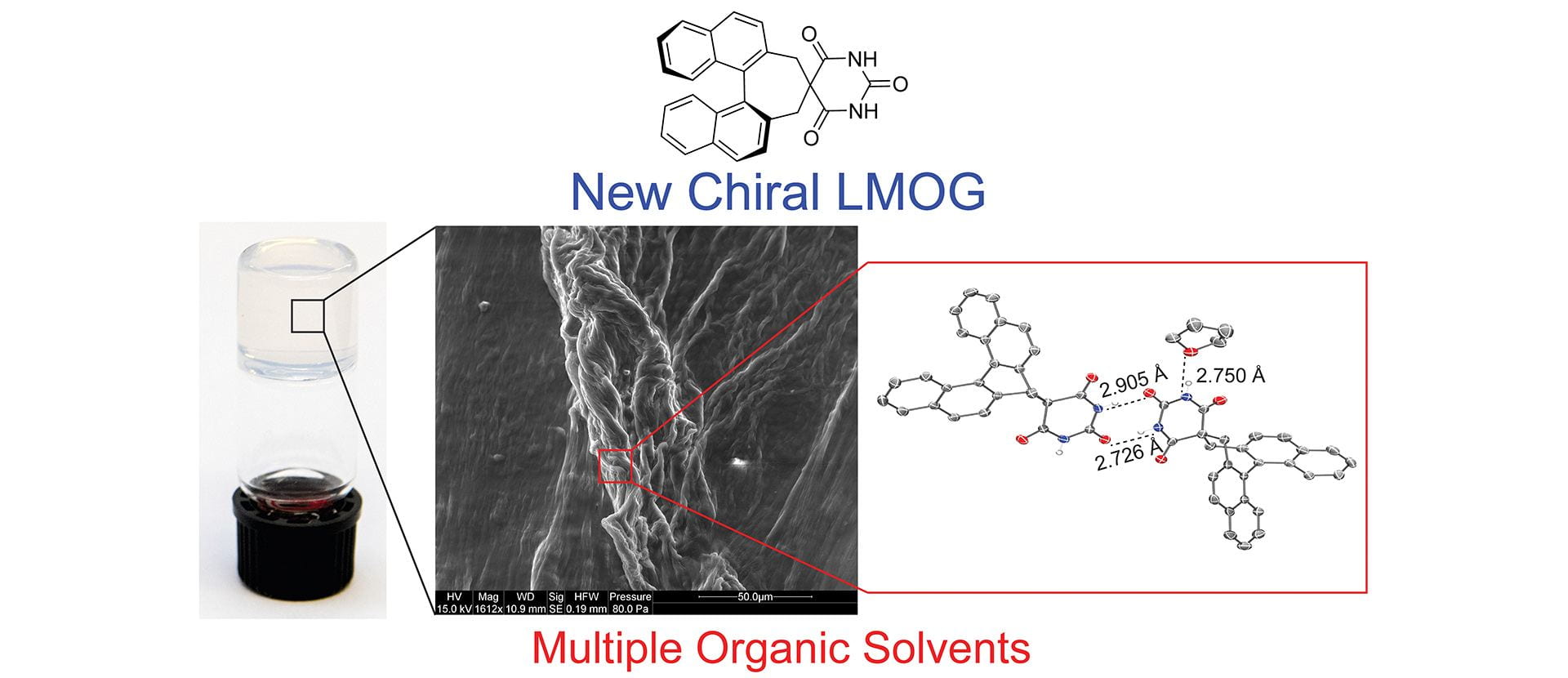
OA23. Seidenkranz, D.T.; Langworthy, K.A.; Zakharov, L.N.; Pluth, M.D. “Single-Component, Low Molecular Weight Organic Supergelators Based on Chiral Barbiturate Scaffolds.” Supramol. Chem. 2019, 31(8), 499-507. [10.1080/10610278.2019.1629437]
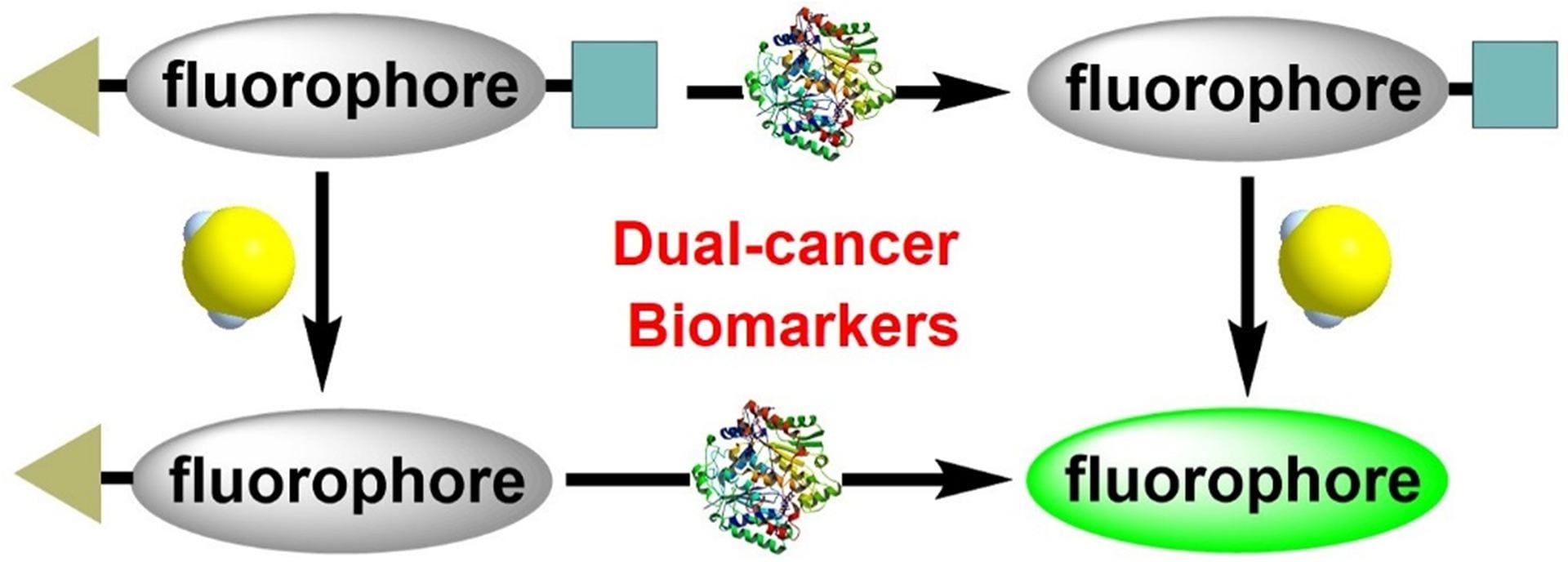
OA22. Zhang, C.; Zhange. Q.Z.; Zhang, K.; Li, L.Y.; Pluth, M.D.; Yi, L. Xi, Z. “Dual-Biomarker-Triggered Fluorescence Probes for Differentiating Cancer Cells and Revealing Synergistic Antioxidant Effects Under Oxidative Stress.” Chem. Sci. 2019, 10, 1945-1952. [10.1039/C8SC03781G]
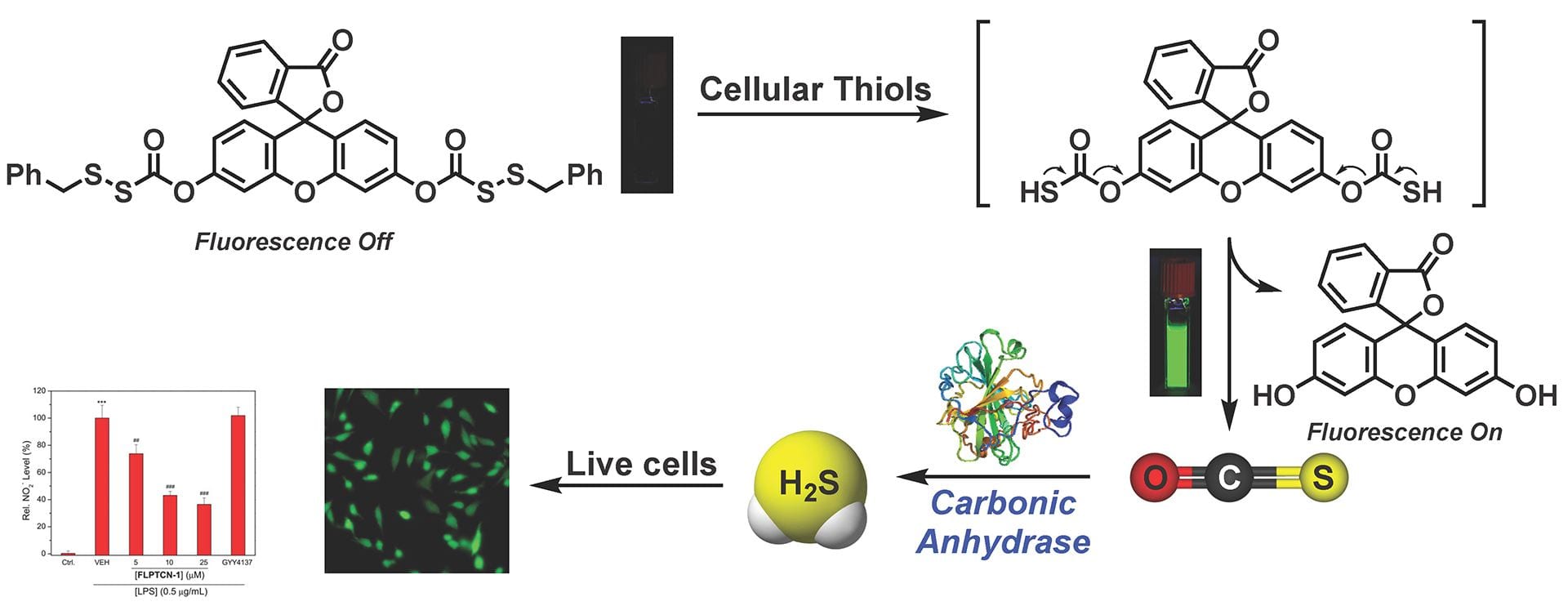
OA 21. Zhao, Y.; Cerda, M.M; Pluth, M.D. “Fluorogenic Hydrogen Sulfide (H2S) Donors Based on Sulfenyl Thiocarbonates Enable H2S Tracking and Quantification ” Chem. Sci. 2019, 10, 1873-1878. [10.1039/C8SC05200J]
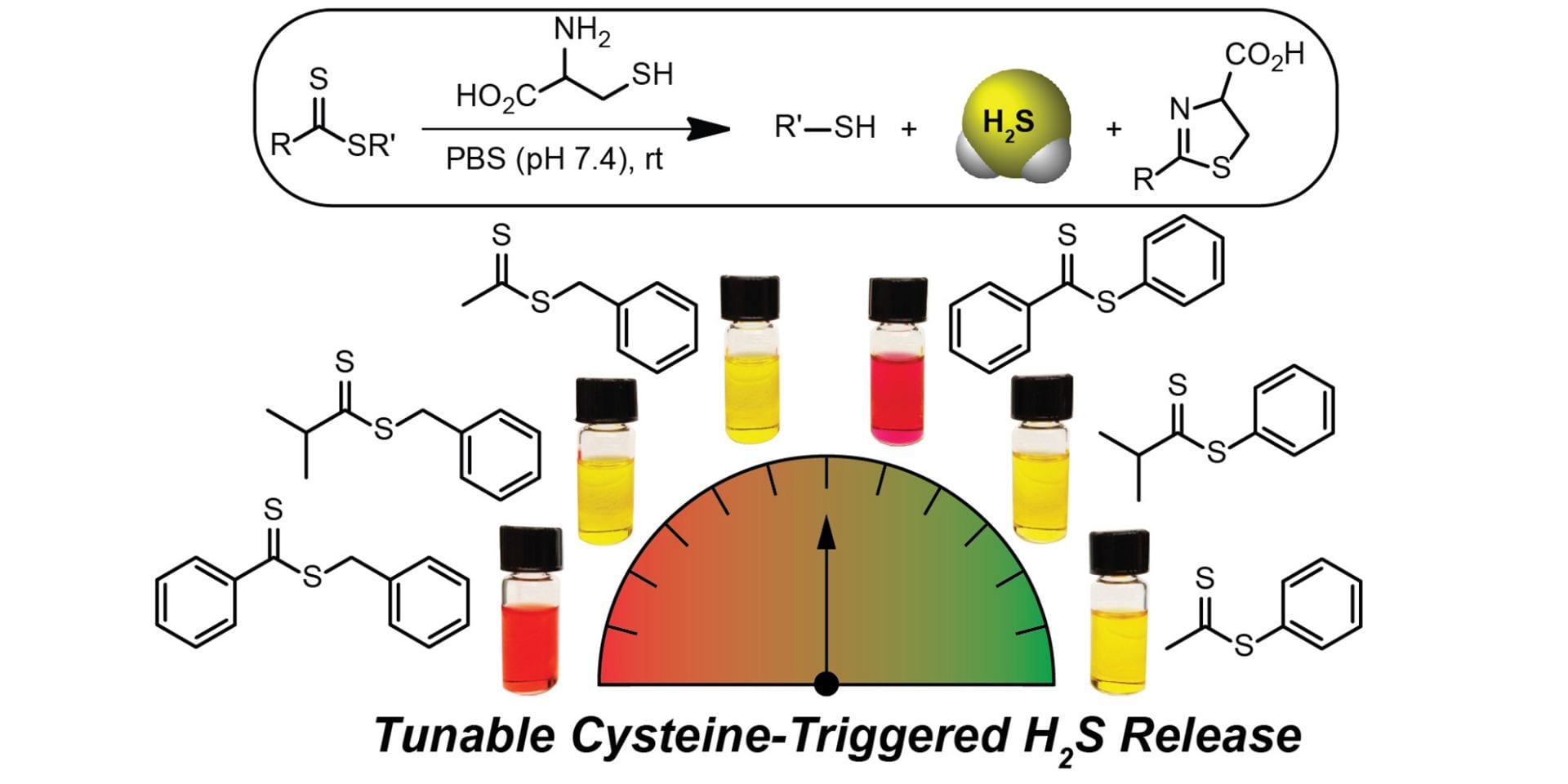
OA 20. Cerda, M.M; Newton, T.D.; Zhao, Y.; Collins, B.K.; Hendon, C.H.; Pluth, M.D. “Dithioesters: Simple, Tunable, Cysteine-Selective H2S Donors” Chem. Sci. 2019, 10, 1773-1779. [10.1039/C8SC04683B]
OA19. Fargher, H.A.; Lau, N.; Zakharov, L.N.; Haley, M.M.; Johnson, D.W.; Pluth, M.D. “Expanding Reversible Chalcogenide Binding: Supramolecular Receptors for the Hydroselenide (HSe–) Anion” Chem. Sci. 2019, 10, 67-72. [10.1039/C8SC03968B]
– Back Cover

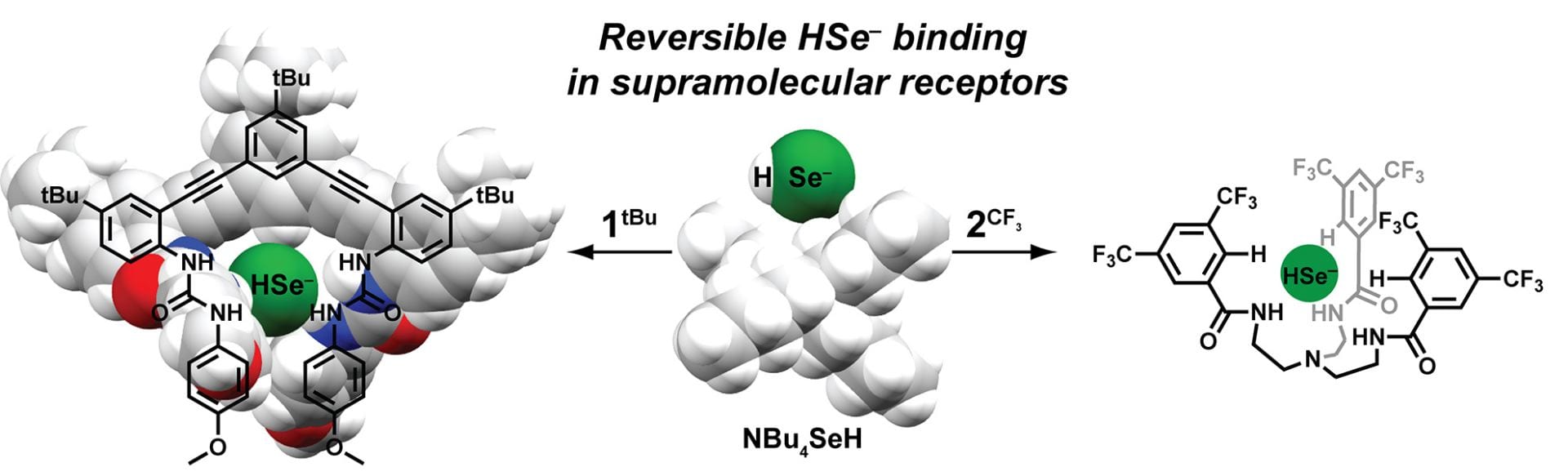
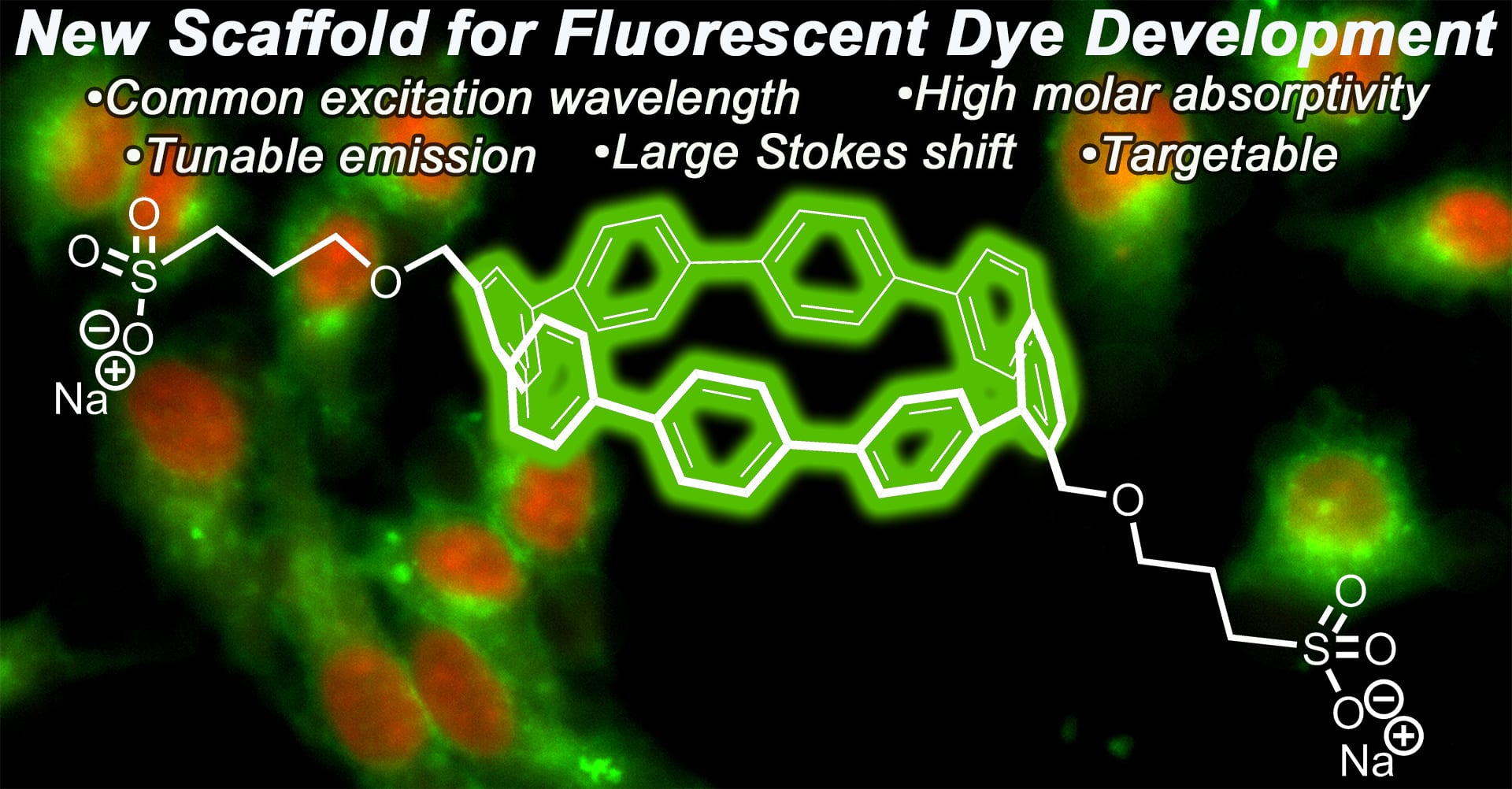
OA18. White, B.M.; Zhao, Y.; Kawashima, T.E.; Branchaud, B.P.; Pluth, M.D.; Jasti, R. “Expanding the Chemical Space of Biocompatible Fluorophores: Nanohoops in Cells.” ACS Cent. Sci. 2018, 4(9), 1173-1178. [10.1021/acscentsci.8b00346]
– Highlighted by [EurekAlert!], [ScienceDaily], [Phys.org], [R&D]
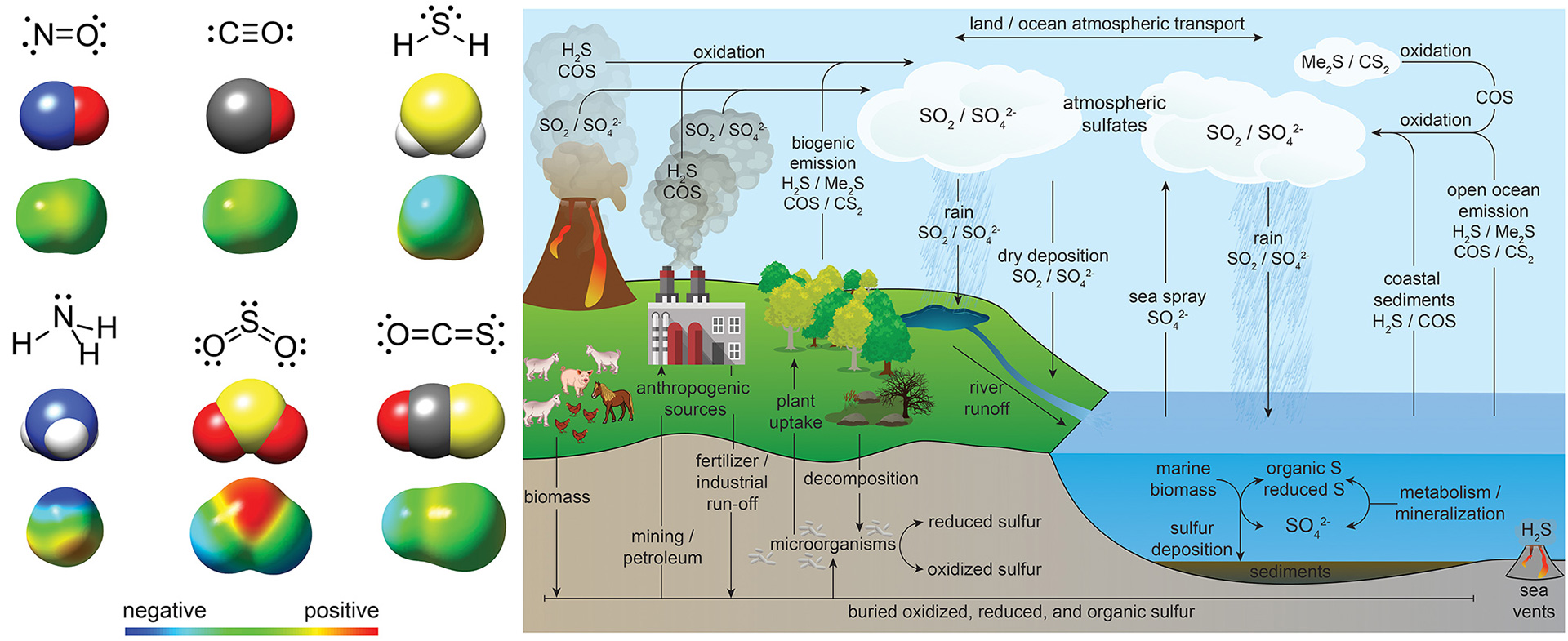
OA17. Steiger, A.K.; Zhao, Y.; Pluth, M.D. “Emerging Roles of Carbonyl Sulfide (COS) in Chemical Biology: Sulfide Transporter or Gasotransmitter?” Antioxid. Redox Signal. 2018, 28(16), 1516-1532. [10.1089/ars.2017.7119]
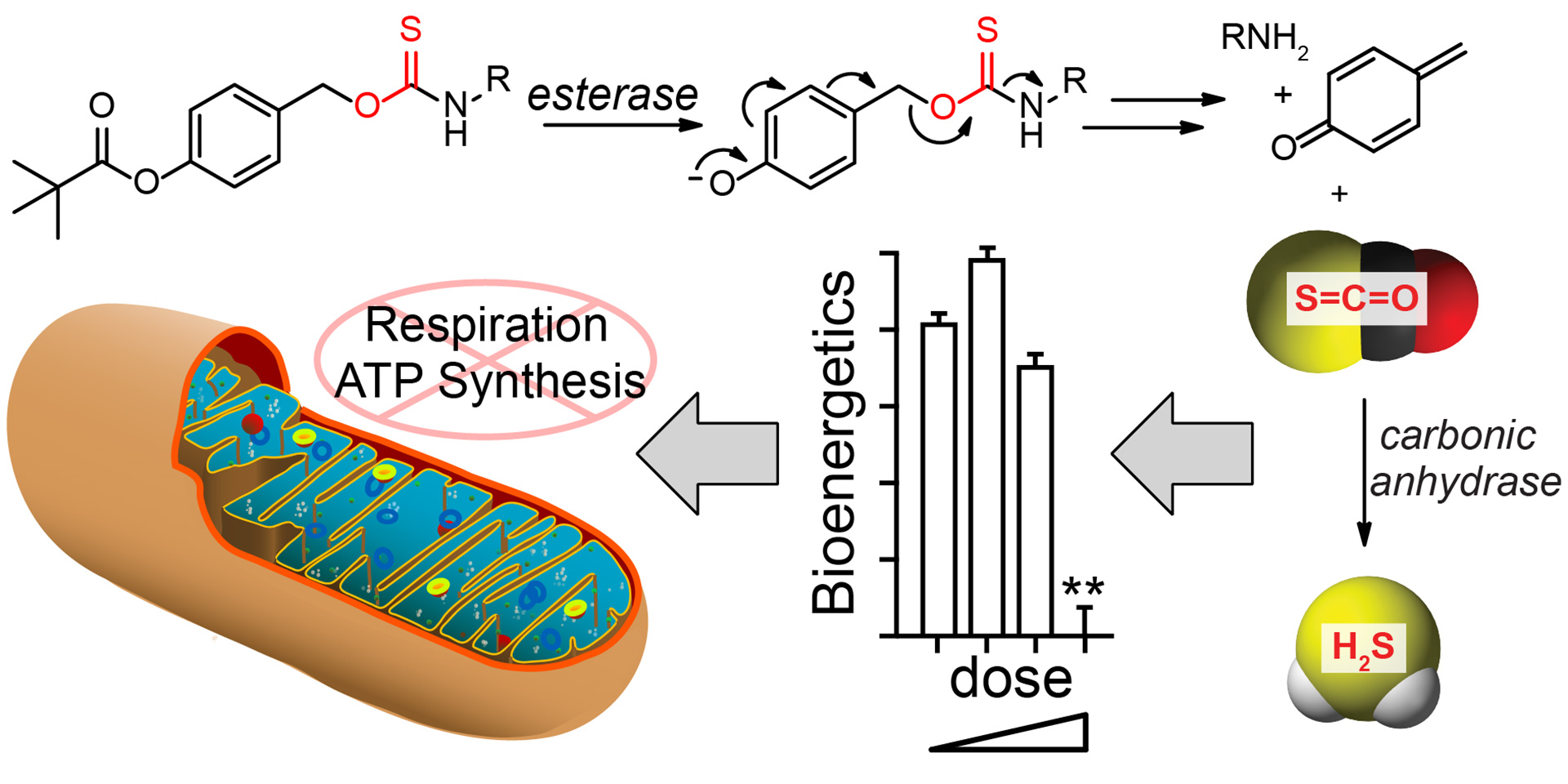
OA16. Steiger, A.K.; Marcatti, M.; Szczesny, B.; Pluth, M.D. “Inhibition of Mitochondrial Bioenergetics by Esterase-Triggered COS/H2S Donors” ACS Chem. Biol. 2017, 12(8), 2117-2123. [10.1021/acschembio.7b00279]
OA15. Cerda, M.M.; Hammer, M.D.; Earp, M.S.; Zakharov, L.N.; Pluth, M.D. “Applications of Synthetic Organic Tetrasulfides as H2S Donors.” Org. Lett. 2017, 19(9), 2314-2317. [10.1021/acs.orglett.7b00858]
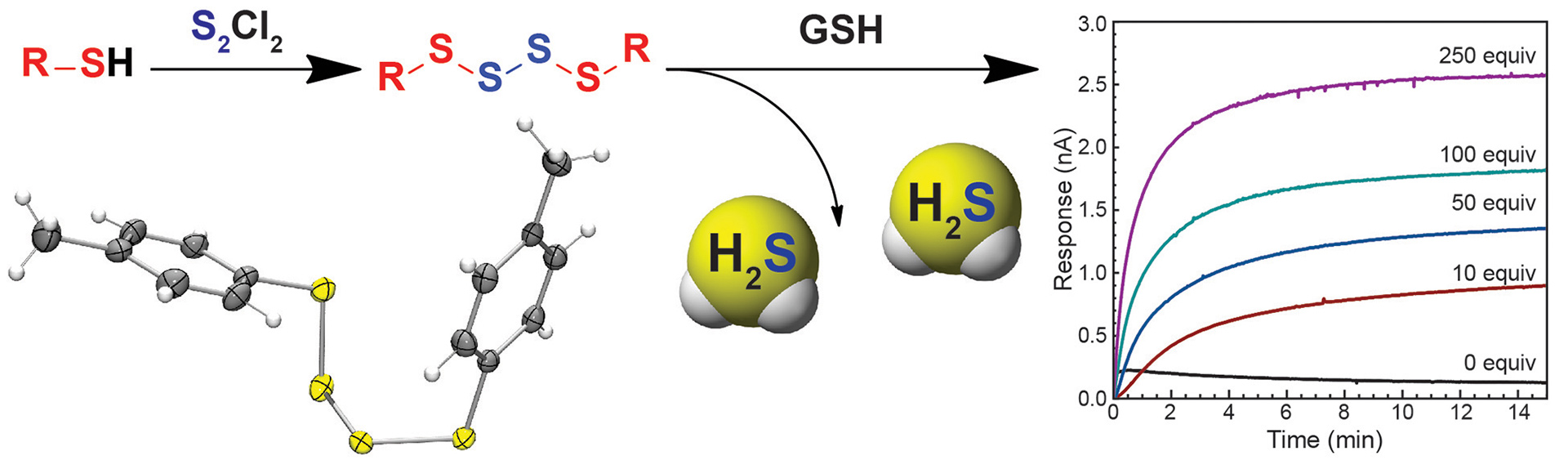
OA14. Zhao, Y.; Bolton, S.G.; Pluth, M.D. “Light-Activated COS/H2S Donation from Photocaged Thiocarbamates. Org. Lett. 2017, 19(9), 2278-2281. [acs.orglett.7b00808]
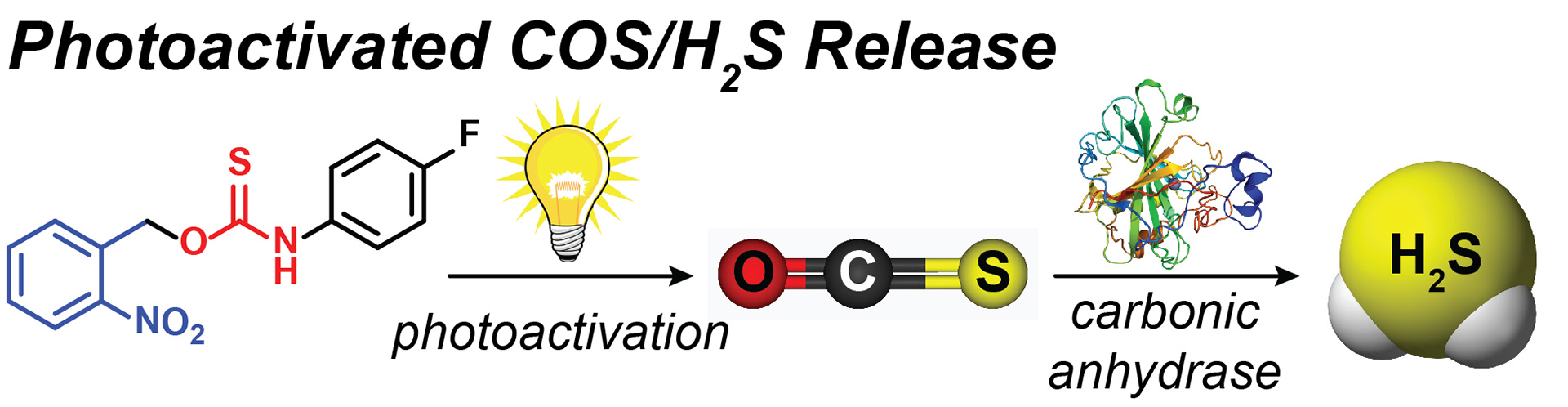
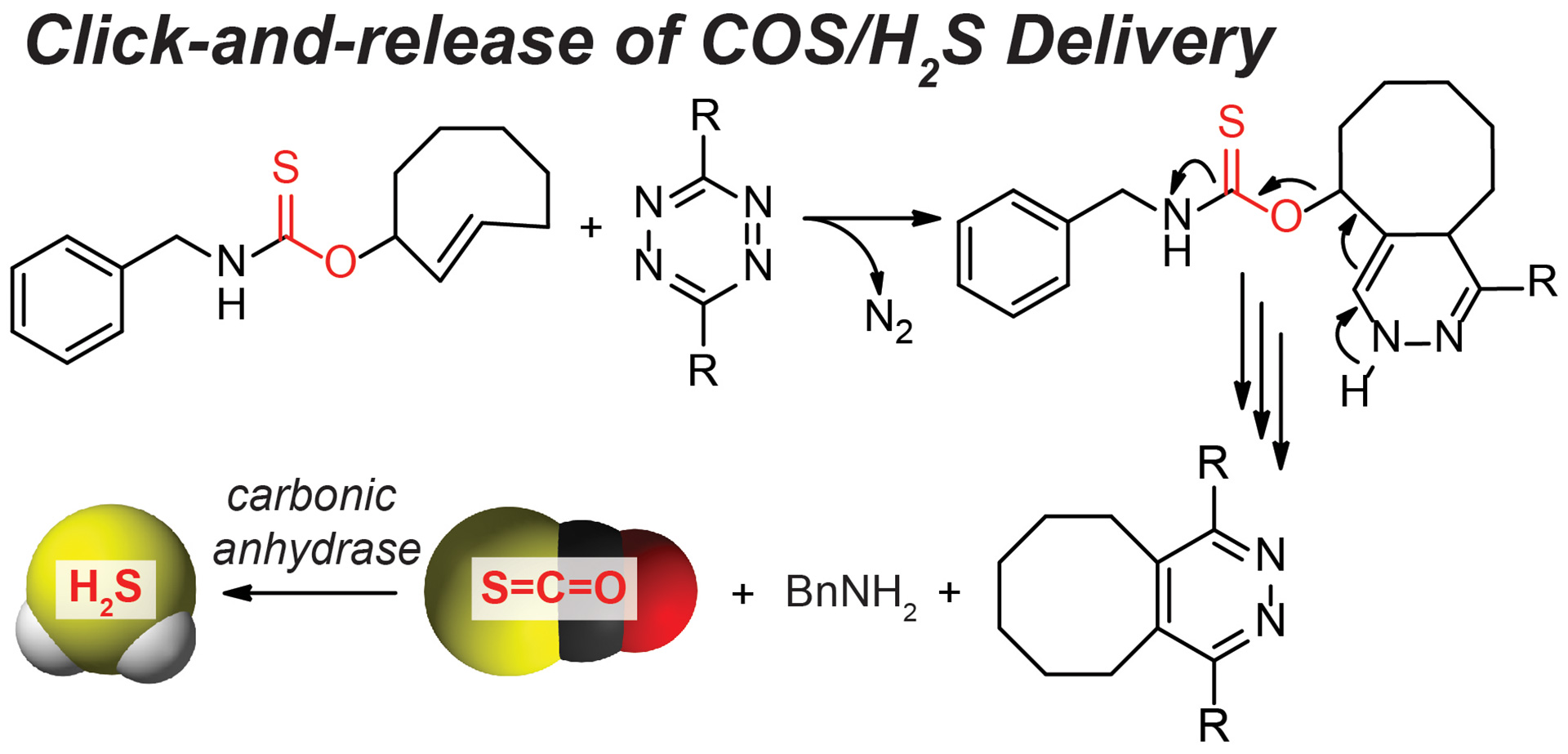
OA13. Steiger, A.K.; Yang, Y.; Royzen, M.; Pluth, M.D. “Bio-orthogonal “Click-and-Release” Donation of Caged Carbonyl Sulfide (COS) and Hydrogen Sulfide (H2S).” Chem. Commun. 2017, 53, 1378-1380. [10.1039/C6CC09547J]
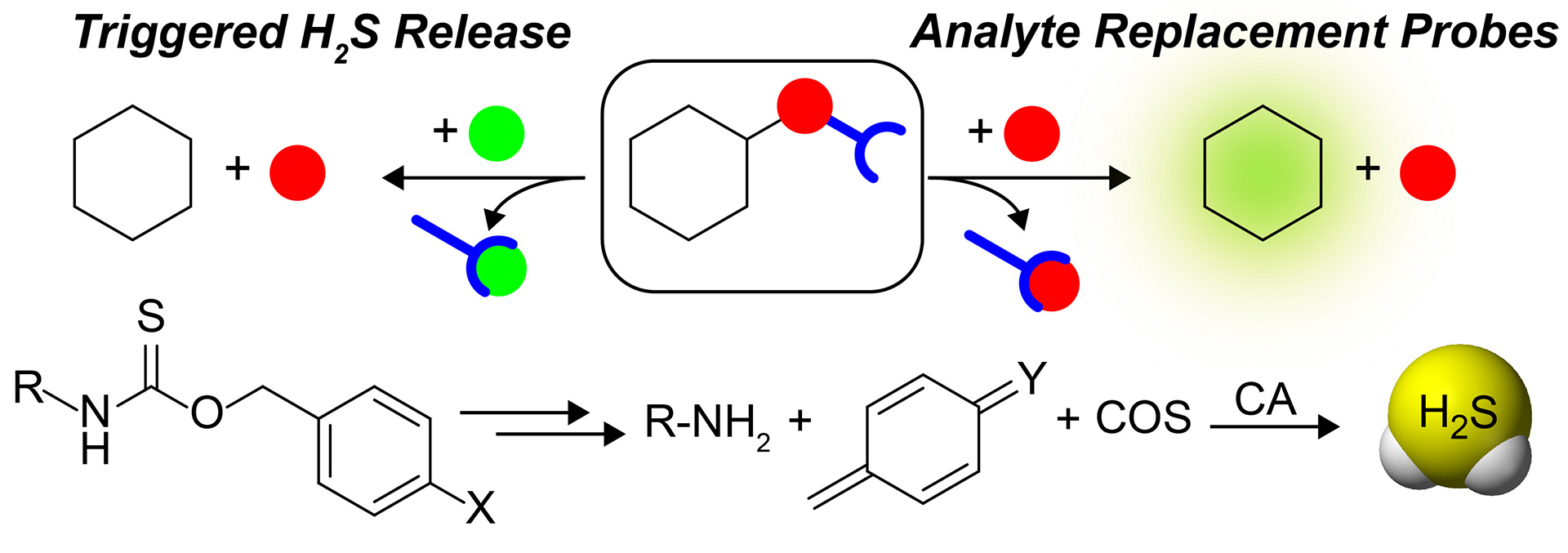
OA12. Steiger, A.K.; Pardue, S.; Kevil, C.G.; Pluth, M.D. “Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes.” J. Am. Chem. Soc. 2016, 138, 7256-7259. [10.1021/jacs.6b03780]
– Highlighted by [AroundTheO], [Phys.org], [ScienceBlog], [EurekaAlert!] [ChemEurope]
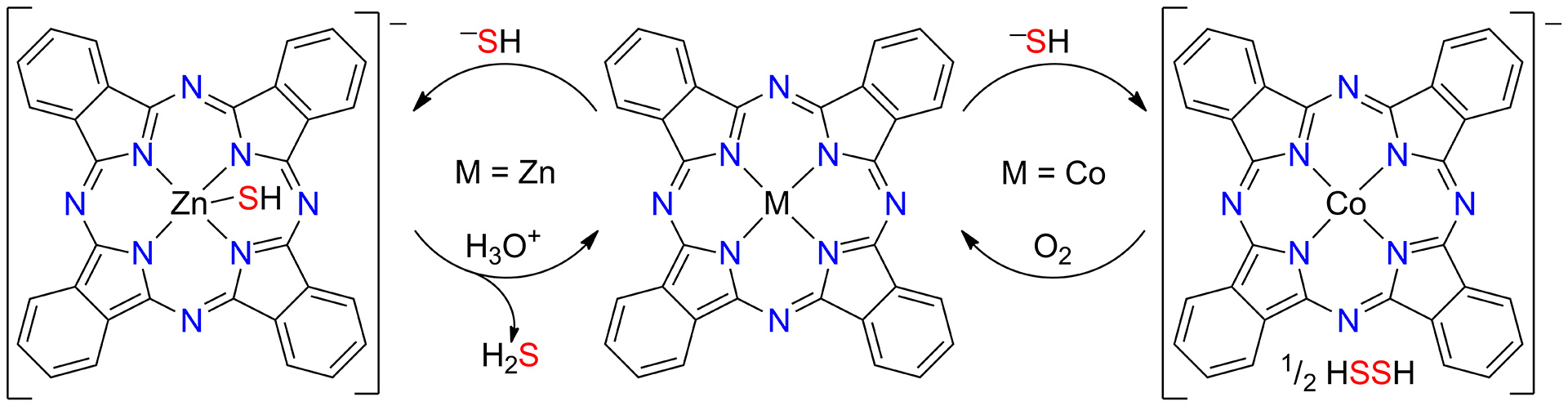
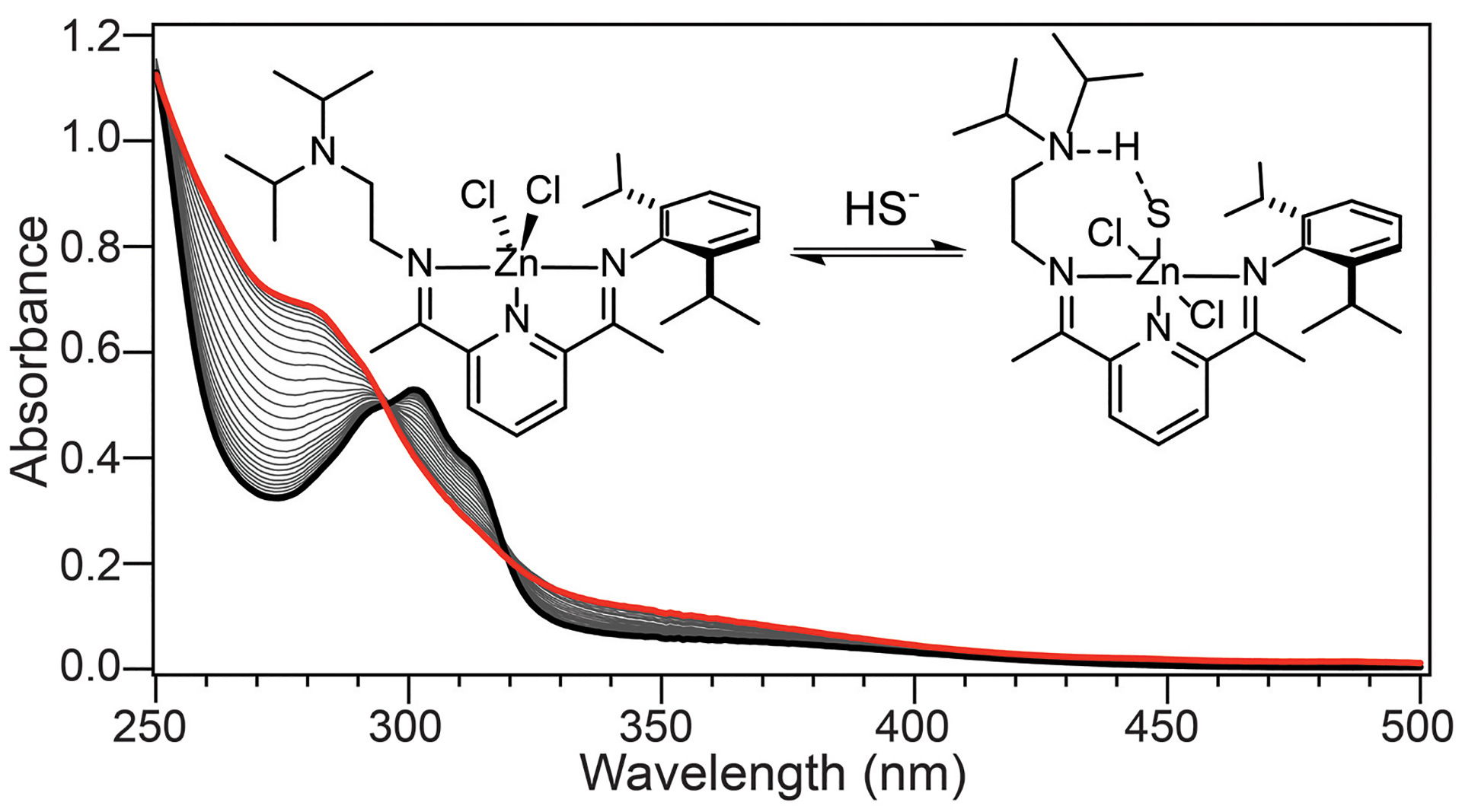
OA11. Hartle, M.D.; Delgado, M.; Gilbertson, J.D.; Pluth, M.D. “Stabilization of a Zn(II) Hydrosulfido Complex Utilizing a Hydrogen-Bond Accepting Ligand.” Chem. Commun. 2016, 52, 7680-7682. [10.1039/C6CC01373B]
OA10. Hartle, M.D.; Pluth, M.D. “A Practical Guide to Working with H2S at the Interface of Chemistry and Biology.” Chem. Soc. Rev. 2016, 45, 6108-6117. [10.1039/C6CS00212A]
– Cover article

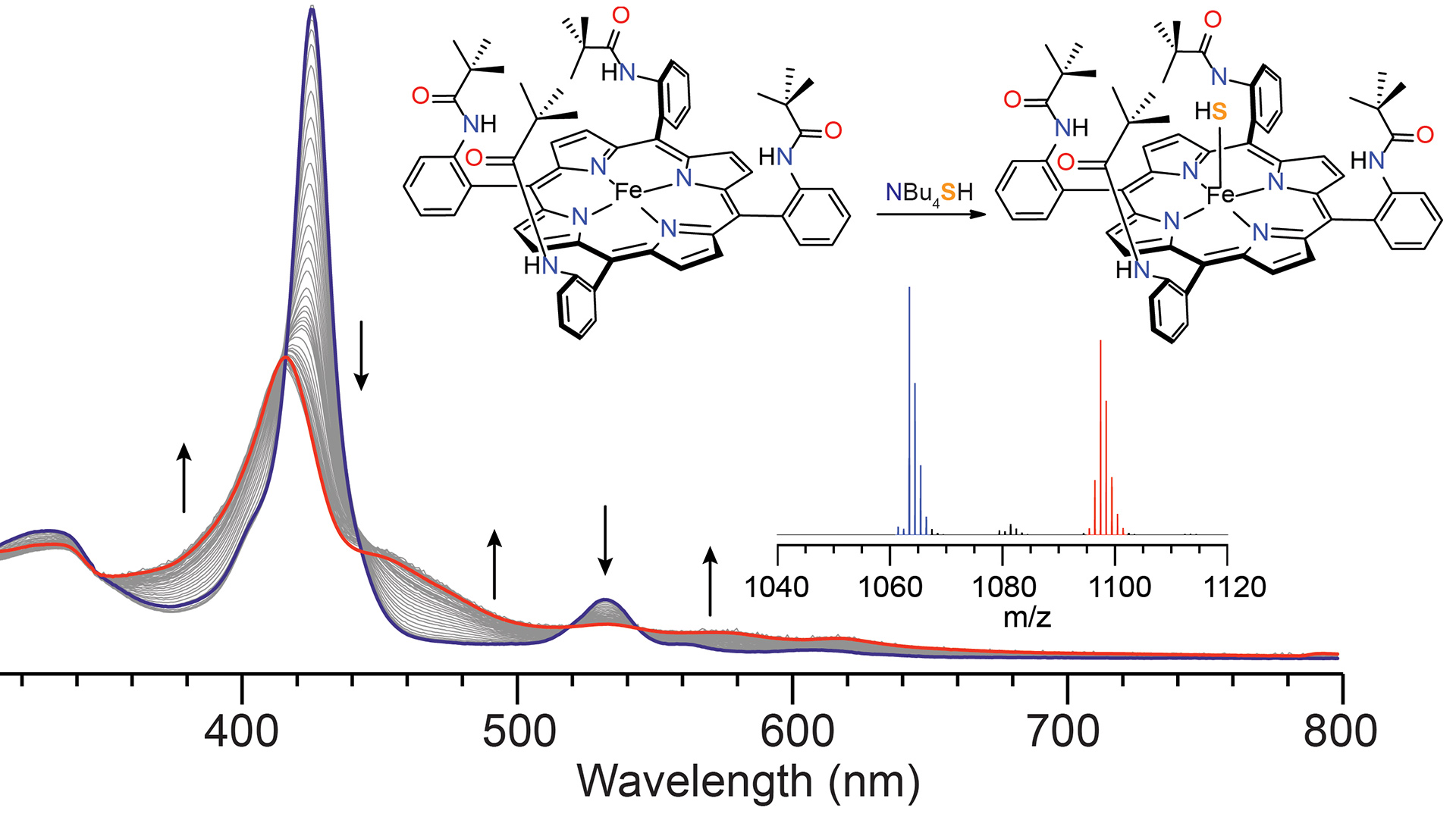
OA9. Hartle, M.D.; Prell, J.S.; Pluth, M.D. “Spectroscopic Investigations into the Binding of Hydrogen Sulfide to Synthetic Picket-Fence Porphyrins.” Dalton Trans. 2016, 45, 4843-4853. [10.1039/C5DT04563K]
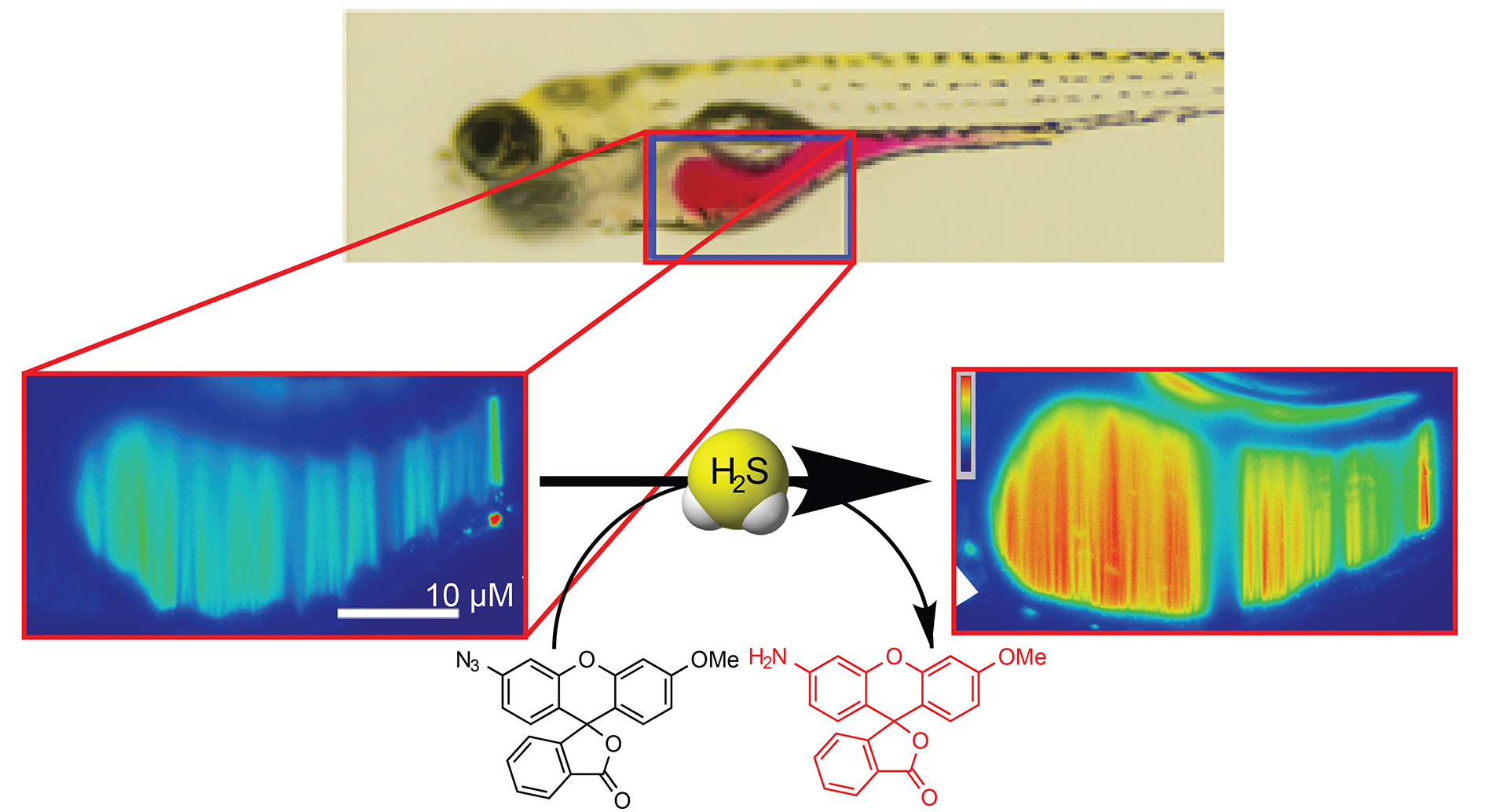
OA8. Hammers, M.D.; Taormina, M.J.; Cerda, M.M.; Montoya, L.A.; Seidenkranz, D.T.; Parthasarathy, R.; Pluth, M.D. “A Bright Fluorescent Probe for H2S Enables Analyte-Responsive, 3D Imaging in Live Zebrafish Using Light Sheet Fluorescence Microscopy.” J. Am. Chem. Soc. 2015, 137(32), 10216-10223. [10.1021/jacs.5b04196]
– JACS Spotlight Article
– Cover article

OA7. Pluth, M.D.; Boettcher, S.W.; Nazin, G.V.; Greenaway, A.L.; Hartle, M.D. “Collaboration and Near-Peer Mentoring as a Platform for Sustainable Science Education Outreach.” J. Chem. Educ. 2015, 92(4), 625–630. [10.1021/ed500377m]
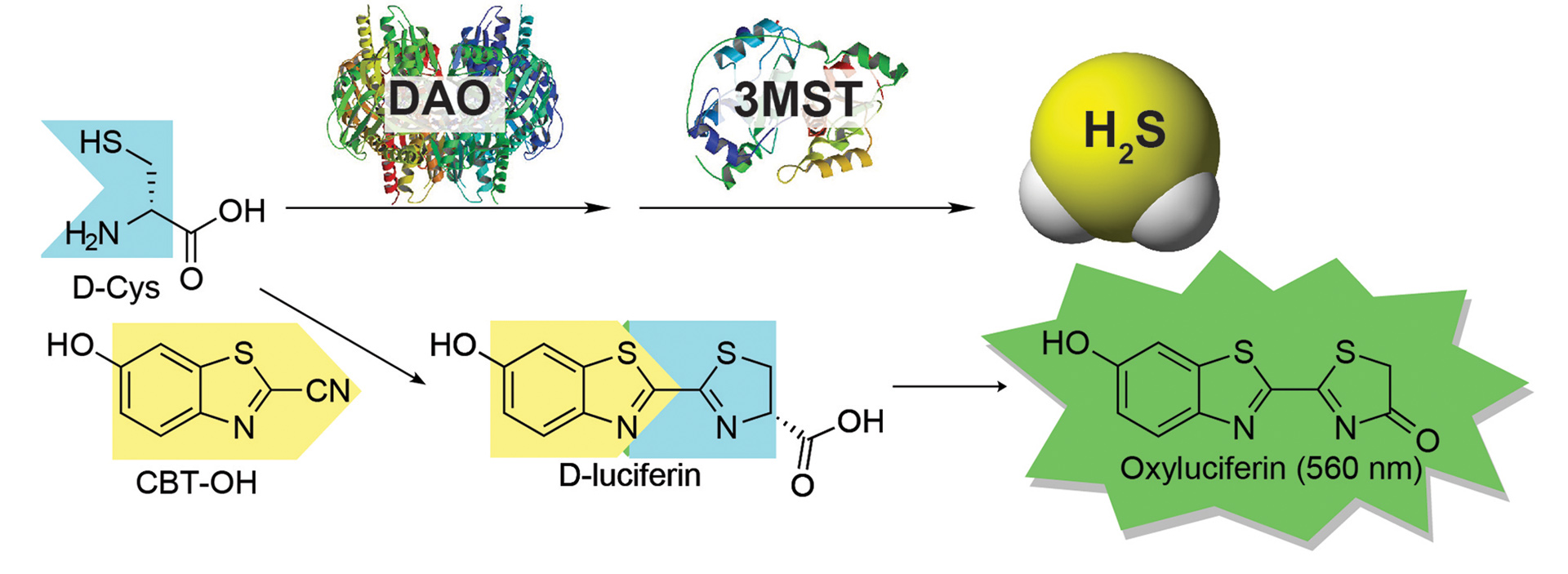
OA6. Bailey, T.S.; Donor, M.T.; Naughton, S.P.; Pluth, M.D. “A Simple Bioluminescent Method for Measuring D-Amino Acid Oxidase Activity.” Chem. Commun. 2015, 51, 5425-5428. [10.1039/c4cc08145e]
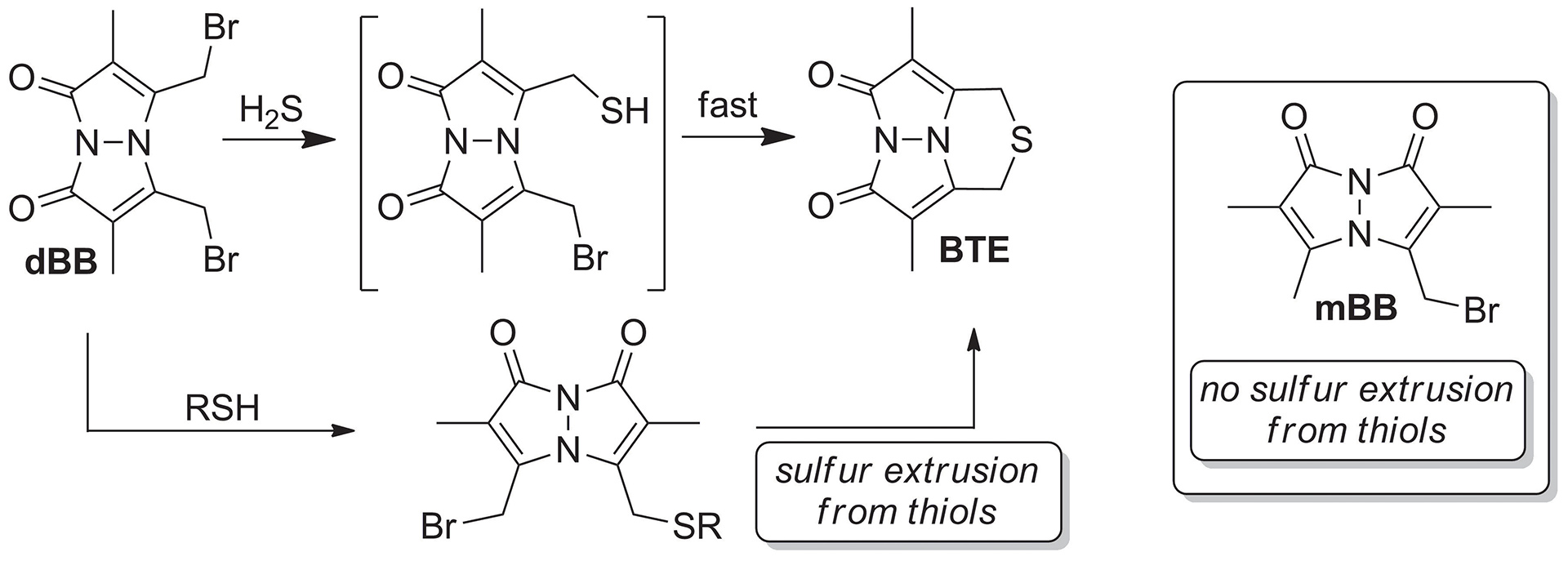
OA5. Montoya, L.A.; Shen, X.; McDermott, J.J.; Kevil, C.G.; Pluth, M.D. “Mechanistic Investigations Reveal that Dibromobimane Extrudes Sulfur from Biological Sulfhydryl Sources other than Hydrogen Sulfide.” Chem. Sci. 2015, 6, 294-300. [10.1039/c4sc01875c]
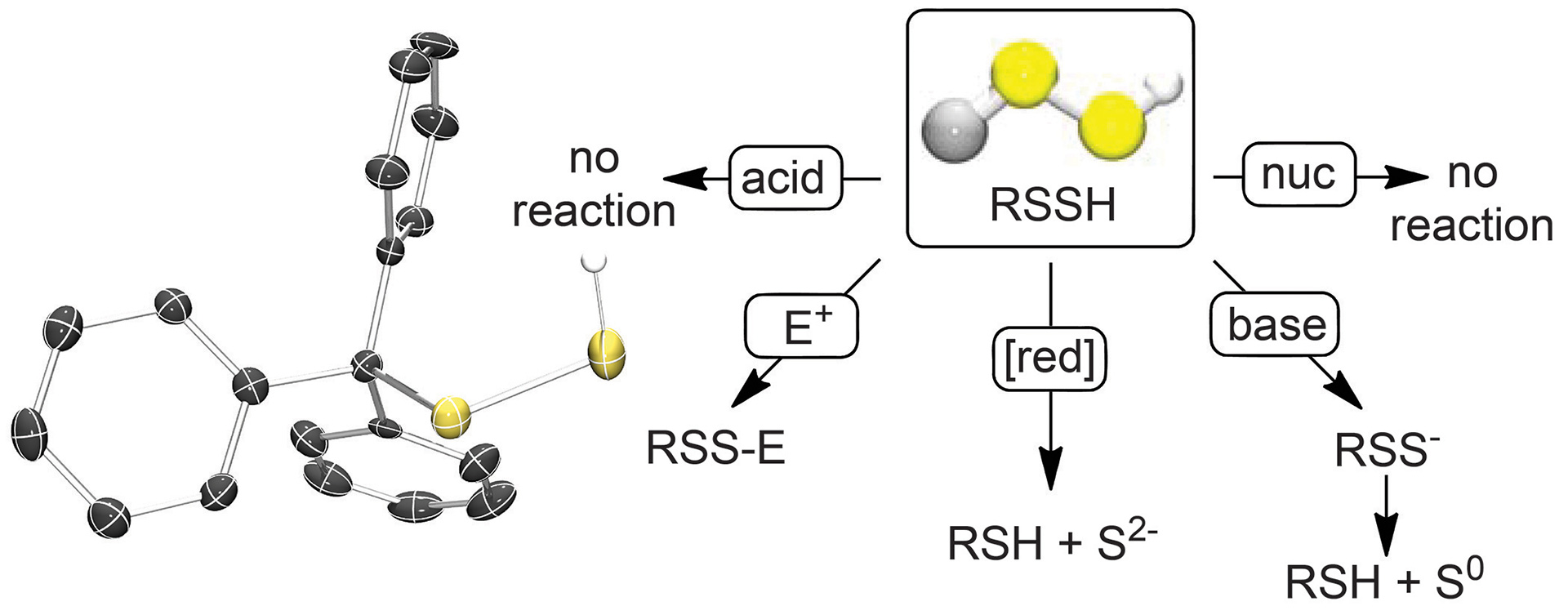
OA4. Bailey, T.S.; Zakharov, L.N.; Pluth, M.D. “Understanding Hydrogen Sulfide Storage: Probing Conditions for Sulfide Release from Hydrodisulfides” J. Am. Chem. Soc. 2014, 136(30), 10573-10576. [10.1021/ja505371z]
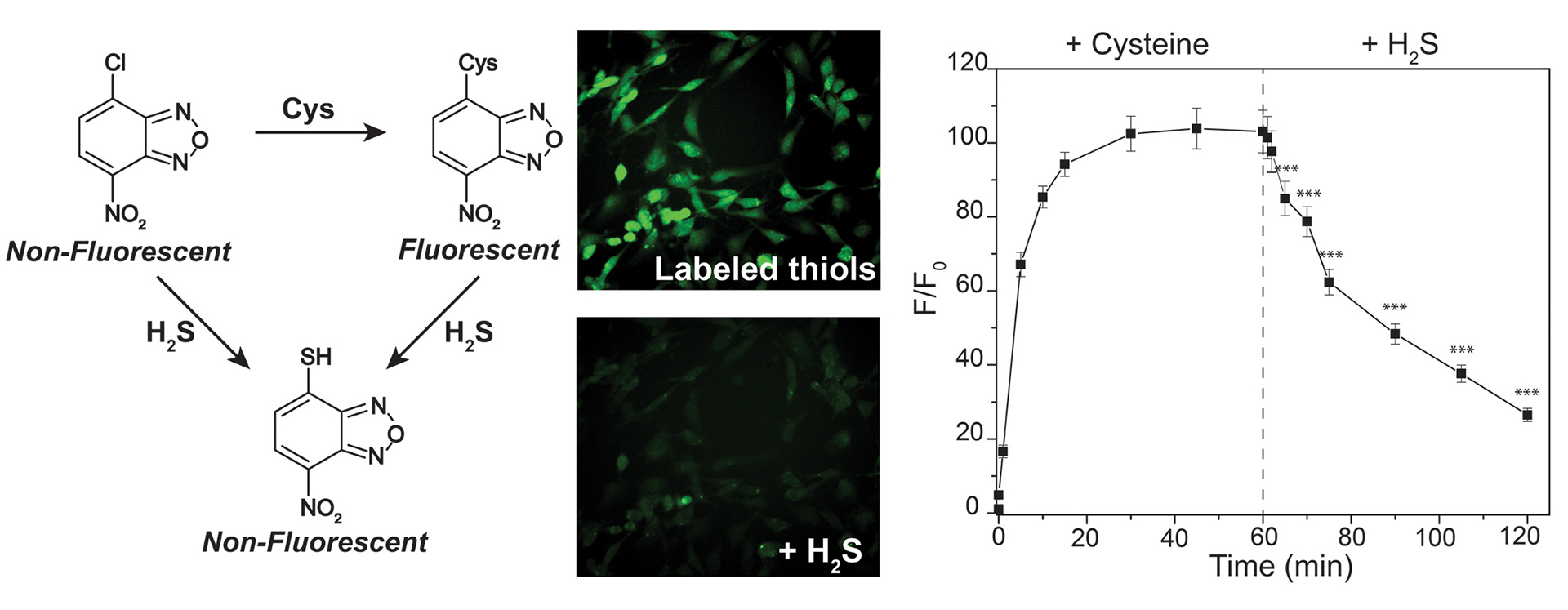
OA3. Hammers, M.D.; Pluth, M.D. “Ratiometric Measurement of Hydrogen Sulfide and Cysteine/Homocysteine Ratios Using a Dual-Fluorophore Fragmentation Strategy” Anal. Chem. 2014, 86(14), 7135–7140.
[10.1021/ac501680d]
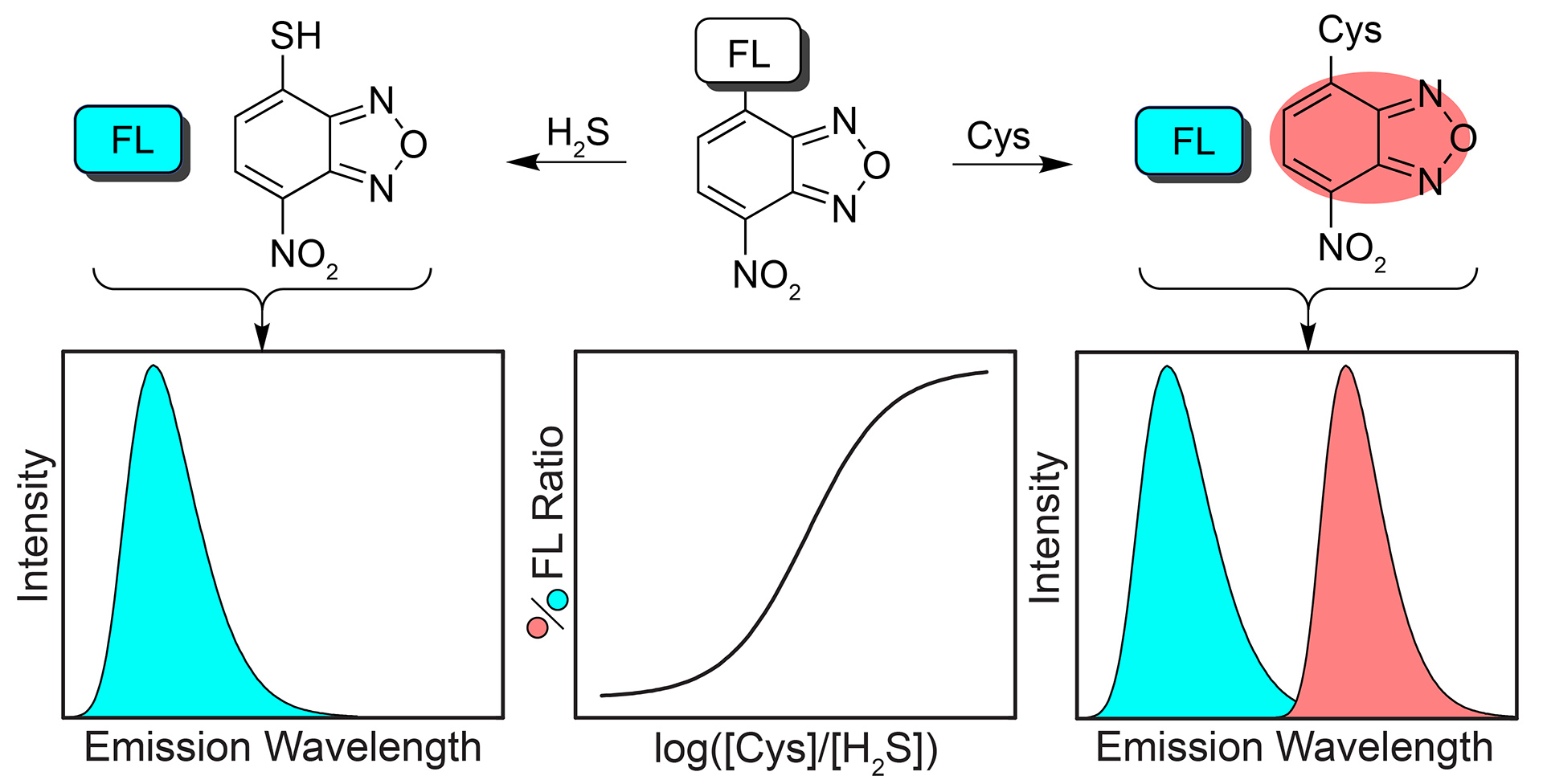
OA2. Montoya, L.A.; Pluth, M.D. “Hydrogen Sulfide Deactivates Common Nitrobenzofurazan-Based Fluorescent Thiol Labeling Reagents.” Anal. Chem. 2014, 86(12), 6032-6039. [10.1021/ac501193r]
OA1. Hartle, M.D.; Sommer, S.K.; Dietrich, S.R.; Pluth, M.D. “Chemically Reversible Reactions of Hydrogen Sulfide with Metal Phthalocyanines” Inorg. Chem. 2014, 53(15), 7800-7802. [10.1021/ic500664c]
– Cover article